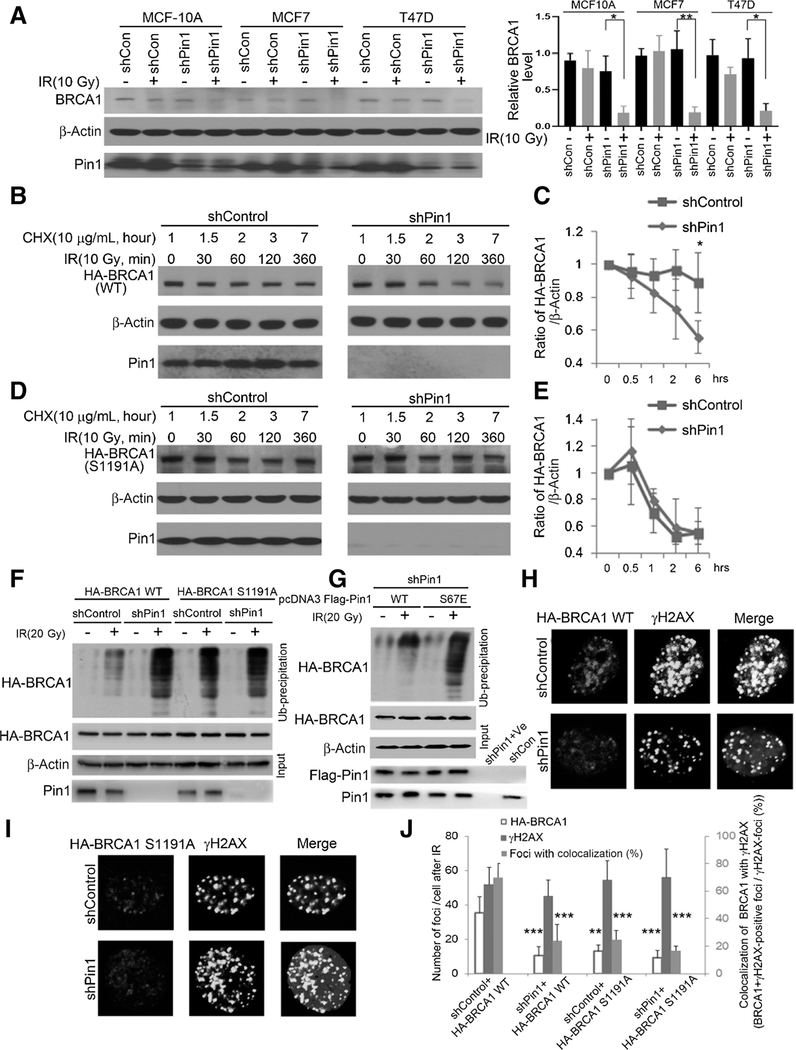
Figure 3.
Pin1-catalyzed prolyl-isomerization is required for radiation-induced stabilization of BRCA1. A, MCF10A, MCF7, and T47D cells were transduced with shRNA against Pin1 or control for 48 hours, then irradiated and lysis 5 hours later. The protein level of BRCA1 was detected by immunoblotting and quantified as ratio relative to β-actin (right). B, MCF7 cells were transfected with HA-BRCA1 and subjected to a cycloheximide (CHX) run-off assay in the absence (right) or presence (left) of Pin1. C, Quantification of HA-BRCA1 stability in B of biological triplicates (the vertical axis starting at 0.4). D, MCF7 cells were transfected with the HA-BRCA1 (S1191A) mutant, identified as Pin1-binding site, and subjected to a cycloheximide run-off assay in the absence (right) or presence (left) of Pin1. E, Quantification of HA-BRCA1 (S1191A) stability in D of biological triplicates (the vertical axis starting at 0.4). F, BRCA1 ubiquitination after irradiation is enhanced in the absence of Pin1 and after extinction of the Serine 1191 phosphorylation site. ShPin1 or ShControl HEK293 cells were transfected with HA-BRCA1 (WT or S1191A) for 48 hours, pretreated with MG132 3 hours prior to irradiation and lysed 6 hours after irradiation. G, The WT, but not the catalytically inactive Pin1 mutant (S67E), reduces BRCA1 ubiquitination. ShPin1 HEK293 cells were transfected with HA-BRCA1 and Flag-Pin1 (WT or S67E mutant) for 48 hours, pretreated with MG132 3 hours prior to irradiation, and lysed 6 hours after irradiation. Absence of Pin1 (H and J) mimics mutation of Serine 1191 (I and J) with regards to DNA repair foci formation. MCF7 cells were transfected with HA-BRCA1 (WT or S1191A) for 48 hours and then irradiated. Immunofluorescence was performed 5 hours after IR. *, P < 0.05; **, P < 0.01; ***, P < 0.001