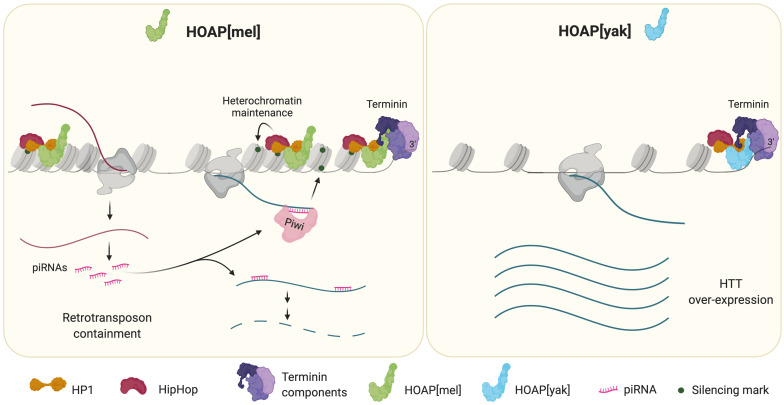
Figure 1. Comparison of telomere phenotypes in Drosophila melanogaster flies expressing HOAP from either D. melanogaster (HOAP[mel]) or D. yakuba (HOAP[yak]).
Left: the telomeres of a D. melanogaster fly expressing HOAP[mel]. The complex formed by HOAP[mel] (green), HipHop (maroon) and HP1 (dark orange) binds the telomere. Bi-directional transcription of the HTT-array produces both full-length transcripts (dark blue lines) and shorter transcripts (bright pink lines) that bind the protein Piwi (light pink) to form piRISC. This complex drives the degradation of the full-length HTT transcripts to prevent retrotransposons from being inserted into other regions of the chromosome. Additionally, piRISC guides the deposition of silencing marks (dark green circles) which serve to recruit HP1 to the telomeres. This leads to chromatin compaction, reducing HTT-array transcription and providing a second layer of control over the retrotransposons. The complex formed by HOAP, HipHop and HP1 also binds to terminin (purple) to cap and protect the chromosome end. Right: telomeres of a D. melanogaster fly expressing the cav gene from D. yakuba, HOAP[yak]. While HOAP[yak] (light blue) can bind to HipHop and HP1 and bind terminin to effectively cap the chromosome ends, it fails to perform the roles of the native HOAP in regulating the HTT-array. This means that the telomeres of flies expressing HOAP[yak] have lower levels of HP1 and do not have silencing marks to aid in the compaction of chromatin. Consequently, chromatin becomes relaxed, increasing the transcription of the HTT-array. The full-length transcripts of the retrotransposons that are produced are not degraded because these telomeres do not support the accumulation piRISC. This accelerates retrotransposon replication, initially elongating the telomeres, but then allowing the elements to travel to other regions of the chromosome, potentially disrupting other genes (figure created using BioRender.com).