Abstract
Patient: Male, 70-year-old
Final Diagnosis: Adrenal insufficiency • diabetes insipidus • lung adenocarcinoma Symptoms: Anorexia • fatigue
Medication: —
Clinical Procedure: —
Specialty: Endocrinology and Metabolic • Neurosurgery • Oncology
Objective:
Unusual clinical course
Background:
Hyponatremia is an electrolyte disorder frequently encountered by clinicians. Secondary adrenal insufficiency due to pituitary metastatic tumors should be considered as an alternative diagnosis when clinicians encounter patients with lung cancer who demonstrate hyponatremia. However, masked central diabetes insipidus should also be considered to prevent critical dehydration when glucocorticoid replacement therapy will be initiated.
Case Report:
A 70-year-old man with advanced lung adenocarcinoma demonstrated high-grade hyponatremia of 122 mmol/L. Magnetic resonance imaging disclosed a metastatic pituitary tumor and endocrinological examinations confirmed panhypopituitarism, including secondary adrenal insufficiency. Hydrocortisone replacement revealed masked diabetes insipidus with elevation of serum sodium levels that reached 151 mmol/L. Desmopressin administration was required to prevent water depletion and to immediately ameliorate the hypernatremia.
Conclusions:
This is the first case report of masked diabetes insipidus that demonstrated high-grade hyponatremia. Secondary adrenal insufficiency can mask the hypernatremia that is a typical manifestation of diabetes insipidus. Physicians should consider adrenal insufficiency and diabetes insipidus due to pituitary metastasis of advanced malignancies, even when they encounter patients with hyponatremia.
MeSH Keywords: Diabetes Insipidus, Adrenal Insufficiency, Glucocorticoids, Hyponatremia, Lung Neoplasms, Pituitary Neoplasms
Background
Metastatic pituitary tumors are observed in approximately 0.1% to 28% of autopsy cases with various malignancies [1]. Among the various malignancies, lung cancer is a common source of metastatic pituitary tumors [1–3]. Actually, approximately 1% of the cases with pituitary tumors in whom transsphenoidal hypophysectomy was performed had metastatic pituitary tumors [1]. In this context, physicians should not overlook occult pituitary metastasis, even when the various symptoms of advanced malignant diseases have cloaked it.
Metastasis is more likely to occur in the posterior lobe than in the anterior lobe of the pituitary gland because the posterior lobe is fed by the pituitary artery, whereas the latter is fed by the pituitary portal vein [2]. Thus, posterior lobe function tends to be disturbed compared with anterior lobe functions. Disturbances of antidiuretic hormone (ADH), adrenocortico-tropic hormone (ACTH), thyroid-stimulating hormone (TSH), gonadotropins (luteinizing hormone [LH] and follicle-stimulating hormone [FSH]), and prolactin (PRL) are observed in 59%, 49%, 57%, 70%, and 83% of patients, respectively, with metastatic pituitary tumors. In contrast, few patients with pituitary adenoma exhibit diabetes insipidus, whereas 26%, 14%, and 49% of them have disturbances in ACTH, TSH, and gonadotropins, respectively [4]. Thus, diabetes insipidus, visual disturbance, symptoms of anterior lobe disturbance, disturbed eye movement, head or eye pain, and general fatigue are frequently observed in patients with metastatic pituitary tumors [1,2,5].
Hyponatremia is an electrolyte disorder that physicians frequently encounter [6,7]. Patients with lung cancer may develop hyponatremia because of 2 endocrinological issues: high intrathoracic pressure-induced syndrome of inappropriate secretion of ADH (SIADH) [8,9] and primary [10] or secondary [10,11] adrenal insufficiency owing to metastatic tumor. With regard to pituitary metastasis, it remains unclear whether patients who have both secondary adrenal insufficiency and central diabetes insipidus because of metastatic tumor could demonstrate hyponatremia.
Here, we report the case of a patient with secondary adrenal insufficiency complicated by central diabetes insipidus because of pituitary metastasis of lung adenocarcinoma. This is the first case report of a patient who demonstrated severe hyponatremia in that condition. Of note, we describe the remarkable changes in serum sodium concentration during the patient’s clinical course. Physicians should be aware of and not overlook this unique condition when they encounter hyponatremia with advanced malignancies.
Case Report
A 70-year-old man was admitted to our hospital complaining of anorexia and hyponatremia during chemotherapy for lung cancer. He had developed lung adenocarcinoma (Figure 1) at the age of 65 and underwent left upper lobectomy. Recurrence in the lung and multiple brain metastases were revealed by computed tomography (CT) and magnetic resonance imaging (MRI), respectively, 16 months prior to the admission, but no abnormalities were noted in the pituitary gland. Gamma knife radiotherapy for the brain metastases and chemotherapy using erlotinib were started. However, exacerbation of the brain metastases was observed with progression of hyponatremia and malaise.
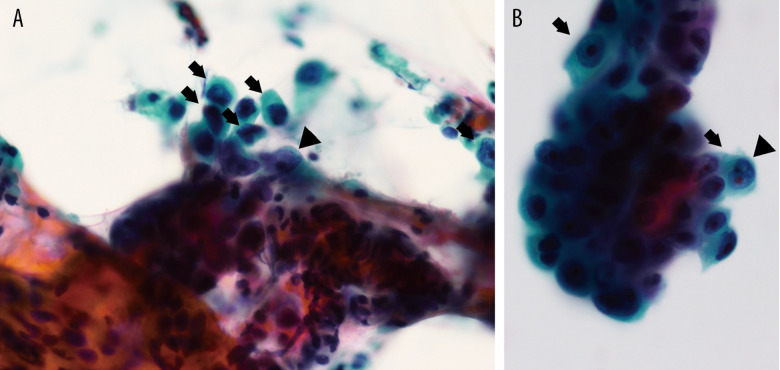
Figure 1.
Pathological examination with trans-bronchial lung biopsy. (A, B) Atypical cells with enlarged and irregular nucleus (arrows) and clear nucleolus (arrow heads) are shown.
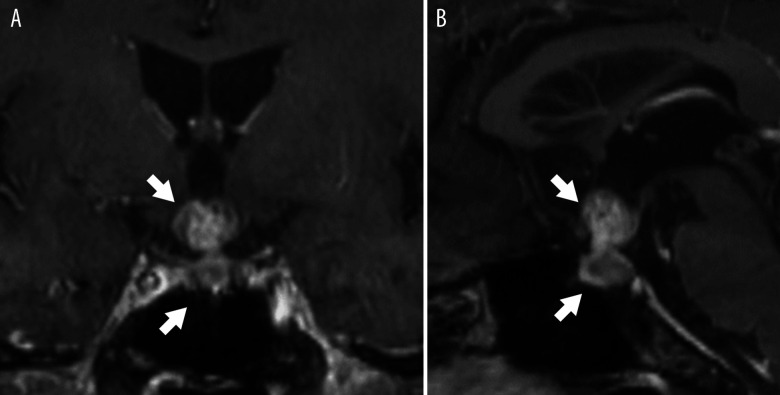
Severe hyponatremia of 122 mmol/L was noted at his first admission on April 27, 2018 (Table 1). Initially, hyponatremia due to anorexia was considered; thus, 6 g/day of sodium chloride was administrated orally and 500 mL/day of saline intravenously. The serum sodium level increased to 135 mmol/L on May 2, 2018, and he was discharged from the hospital; however, the hyponatremia reappeared. SIADH was also considered because of the preserved urinary sodium level (41 mmol/mL) and the high osmolality of the urine compared with the low osmolality of the plasma; however, further examinations of endocrine function disclosed a decrease in the levels of ACTH, FSH, LH, and TSH (Table 1). Additionally, MRI revealed pituitary metastasis of the lung adenocarcinoma (Figure 2). We could not assess the high-intensity signal in T1-weighted image of the posterior lobe because of restricted availability of enhanced imaging and severe plasma hypoosmolality. Thus, we diagnosed panhypopituitarism, and 20 mg/day of hydro-cortisone and 50 μg/day of levothyroxine were administered for replacement therapy (Figure 3).
Table 1.
Results of laboratory examinations performed at admission.
| Parameters | Value | Reference range |
|---|---|---|
| Chemistry | ||
| Blood urea nitrogen (mg/dL) | 20 | 8–21 |
| Creatinine (mg/dL) | 0.82 | 0.65–1.07 |
| Uric acid (mg/dL) | 4.0 | 3.7–7.0 |
| Sodium (mmol/L) | 128 | 138–145 |
| Potassium (mmol/L) | 4.4 | 3.6–4.8 |
| Chlorine (mmol/L) | 92 | 101–108 |
| Plasma osmotic pressure (mOsm/kg•H2O) | 292 | 275–290 |
| Urine osmolality (mOsm/kg•H2O) | 175 | 50–1300 |
| Endocrinology | ||
| Adrenocorticotropic hormone (pg/mL) | 1.1 | 7.2–63.3 |
| Cortisol (μg/dL) | 1.67 | 6.24–18.00 |
| Dehydroepiandrosterone sulfate (μg/dL) | 6 | 18–391 |
| Urine free cortisol (mg/d) | ≤12.5 | 11.2–80.3 |
| Arginine vasopressin (pg/mL) | 0.5 | <2.8 |
| Thyroid-stimulating hormone (μIU/mL) | 1.440 | 0.500–5.000 |
| Free thyroxine (ng/dL) | 0.49 | 0.93–1.70 |
| Luteinizing hormone (IU/mL) | <0.3 | 0.79–5.72 |
| Follicle-stimulating hormone (IU/mL) | 1.3 | 2.00–8.30 |
| Free testosterone (pg/mL) | <0.2 | 0.13–9.88 |
| Growth hormone (ng/mL) | 0.98 | ≤0.64 |
| Insulin-like growth factor-1 (ng/mL) | 14 | 58–198 |
| Plasma renin activity (ng/mL/h) | ≤0.1 | 0.2–2.3 |
| Plasma aldosterone concentration (pg/mL) | 15.1 | 29.9–159.0 |
Figure 2.
Head magnetic resonance imaging. Enhanced dumbbell-type pituitary tumor with suprasellar extension (arrows). (A) Frontal section and (B) sagittal section are shown.
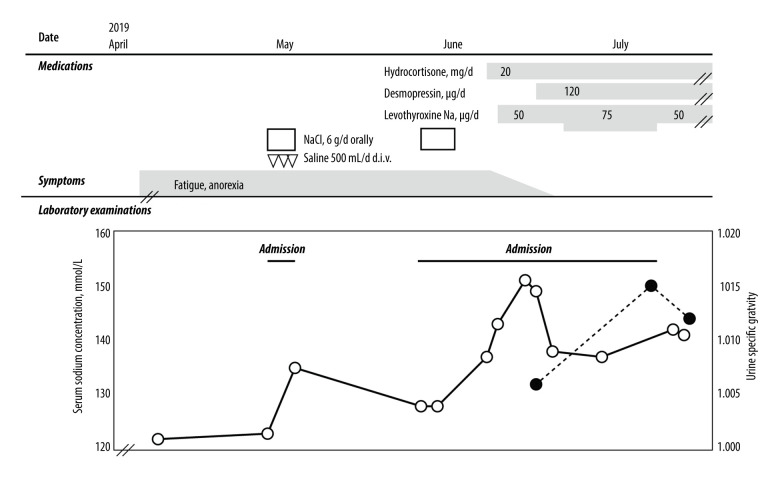
Figure 3.
Clinical course of the present case. Administration of oral and intravenous sodium chloride transiently increased the serum sodium concentration at first admission; however, fatigue and anorexia persisted. Hydrocortisone administration followed by levothyroxine replacement increased the sodium concentration up to 151 mmol/L. Desmopressin administration was required to attenuate the hypernatremia caused by the masked diabetes insipidus. Open circles with solid line represent serum sodium concentration, and closed circles with broken line represent urine specific gravity.
Notably, the hyponatremia was immediately improved and the serum sodium concentration increased from a low level to a high level of 151 mmol/L (Figure 3). Low serum ADH level, high plasma osmolality, low urine osmolality (Table 2), and polyuria with an increased number of urinations (up to 18 times/day) were present; daily urine volumes were not recorded. These findings indicated central diabetes insipidus due to a meta-static pituitary tumor, which had become apparent with corticosteroid administration. Oral desmopressin administration ameliorated the polyuria, increased the urine osmolality, and decreased the serum sodium concentration. Although the panhypopituitarism and diabetes insipidus were regulated by medications, the patient died because of progression of the lung adenocarcinoma 5 months after the first admission.
Table 2.
Results of laboratory examinations before and after desmopressin (DDAVP) initiation.
| Parameters | Value | Reference range | |
|---|---|---|---|
| Before DDAVP | After DDAVP | ||
| Chemistry | |||
| Aspartate aminotransferase (IU/L) | 48 | 26 | 13–30 |
| Alanine aminotransferase (IU/L) | 66 | 29 | 7–30 |
| γ-glutamyl transpeptidase (IU/L) | 29 | 29 | 9–32 |
| Blood urea nitrogen (mg/dL) | 20 | 21 | 8–21 |
| Creatinine (mg/dL) | 0.82 | 0.48 | 0.65–1.07 |
| Sodium (mmol/L) | 149 | 143 | 138–145 |
| Potassium (mmol/L) | 3.5 | 3 | 3.6–4.8 |
| Chlorine (mmol/L) | 109 | 104 | 101–108 |
| Urine osmolality (mOsm/kg•H2O) | 213 | N.A. | 50–1300 |
| Urine specific gravity | 1.006 | 1.015 | 1.006–1.030 |
| Endocrinology | |||
| Plasma renin activity (ng/mL/h) | ≤0.1 | N.A. | 0.2–2.3 |
| Active renin concentration (pg/mL) | ≤2.0 | ≤0.1 | 1.2–35.4 |
| Arginine vasopressin (pg/mL) | <0.4 | 0.5 | <2.8 |
| Plasma osmotic pressure (mOsm/kg•H2O) | 300 | 289 | 275–290 |
High plasma osmolality with low level of arginine vasopressin was noted, and the patient was diagnosed with central diabetes insipidus. DDAVP treatment increased urine osmolality and specific gravity and decreased the plasma osmotic pressure and serum sodium concentration.
Discussion
We described a patient with lung cancer and pituitary metastasis who demonstrated notable changes in serum sodium concentration during his clinical course. This case report demonstrates 2 clinically important issues. First, masked diabetes insipidus can present as severe hyponatremia when complicated by adrenal insufficiency. Second, reevaluation of head MRI is required to detect the occult adrenal insufficiency caused by the pituitary metastasis. Precise evaluation and hormone replacements are required to provide better quality of life (QOL) and prognosis for patients with advanced malignancies.
Masked diabetes insipidus should be considered even when corticosteroid replacement therapy is applied to a patient with secondary adrenal insufficiency exhibiting severe hyponatremia. Diabetes insipidus ordinarily features hyperosmotic hypernatremia, reflecting the deficit of free water [3,7]. There has been no previous case report of diabetes insipidus that showed hyponatremia <130 mmol/L [2,3,5], although a low-normal serum sodium level of masked diabetes insipidus caused by central nervous system sarcoidosis has been reported [12]. Endocrinological examination suggested secondary adrenal insufficiency, secondary hypothyroidism, hypogonadotropic hypogonadism, and growth hormone deficiency in this case. MRI revealed a dumbbell-type tumor of the pituitary gland with suprasellar extension. Corticosteroid replacement led to an increase in urination that probably indicated polyuria, a decrease in the plasma ADH level, and an increase in the serum sodium concentration. Adrenal insufficiency, which has been suggested to induce hyponatremia through a decrease in miner-alocorticoid activity, impaired renal free-water clearance, and a relative increase in ADH secretion, can mask diabetes insipidus [2,5,12]. In this context, although corticosteroid replacement therapy rapidly ameliorates the adrenal insufficiency-related disorders [13], masked diabetes insipidus can be revealed with corticosteroid administration [5,12]. This study alerts physicians that masked diabetes insipidus can develop even in a patient with hypoosmotic hyponatremia.
Reevaluation of head MRI is required to detect the occult adrenal insufficiency due to the pituitary metastasis that is hidden behind the advanced malignancies. Patients with advanced malignancies often have general malaise, appetite loss, and nausea, and these are the major complaints of adrenal insufficiency and hyponatremia [14]. Secondary adrenal insufficiency may show few signs allowing recognition of the abnormality [14], whereas primary adrenal insufficiencies give clues to their diagnosis, including changes in skin pigmentation and elevated plasma ACTH concentrations [15]. Simultaneous evaluation of plasma ACTH paired with serum cortisol is required; however, at baseline, both values are insufficient to establish a diagnosis of secondary adrenal insufficiency in most cases. Further evaluations using stimulations with corticotropin-releasing hormone and/or ACTH are required to confirm the presence of secondary adrenal insufficiency [16]. Patients with metastatic pituitary tumors frequently develop diabetes insipidus, which can also mask the hyponatremia due to adrenal insufficiency [5]. Reevaluation by head MRI of patients with advanced malignancies was suggested to be beneficial to guide proper diagnosis.
Precise evaluation and replacement may be required to provide better QOL and prognosis for patients with advanced malignancies. Advances in chemotherapies, including molecular- and immune-targeting drugs, have recently improved the prognosis of various malignancies [17]. However, these beneficial drugs also have endocrinological adverse reactions [18,19]. Additionally, various malignancies, especially lung carcinoma, have a high frequency of pituitary metastasis. In this context, physicians should evaluate the pituitary functions of patients with lung cancer to avoid critical conditions.
Conclusions
Masked diabetes insipidus should be considered even in a patient with severe hypoosmotic hyponatremia. Pituitary metastasis should not be overlooked, while cachexia of advanced malignancies may mask the adrenal insufficiency. Reevaluation of the head MRI is required to detect occult pituitary metastasis. Precise evaluation and replacement therapy may provide better QOL and prognosis for patients with advanced malignancies.
Acknowledgments
The authors would like to thank Enago (www.enago.jp) for the English language review.
Footnotes
Department and Institution where work was done
Department of Internal Medicine, Kurume University School of Medicine, Kurume, Fukuoka, Japan
Conflicts of interest
None.
References:
- 1.Komninos J, Vlassopoulou V, Protopapa D, et al. Tumors metastatic to the pituitary gland: Case report and literature review. J Clin Endocrinol Metab. 2004;89:574–80. doi: 10.1210/jc.2003-030395. [DOI] [PubMed] [Google Scholar]
- 2.Javanbakht A, D’Apuzzo M, Badie B, Salehian B. Pituitary metastasis: A rare condition. Endocr Connect. 2018;7(10):1049–57. doi: 10.1530/EC-18-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sirinvaravong S, Vibhatavata P, Chunharojrith P, et al. Diabetes insipidus and panhypopituitarism as a first presentation of silent adenocarcinoma of lung: A case report and literature review. BMC Endocr Disord. 2019;19(1):114. doi: 10.1186/s12902-019-0445-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Aridi R, El Sibai K, Fu P, et al. Clinical and biochemical characteristic features of metastatic cancer to the sella turcica: An analytical review. Pituitary. 2014;17(6):575–87. doi: 10.1007/s11102-013-0542-9. [DOI] [PubMed] [Google Scholar]
- 5.Castle-Kirszbaum M, Goldschlager T, Ho B, et al. Twelve cases of pituitary metastasis: A case series and review of the literature. Pituitary. 2018;21(5):463–73. doi: 10.1007/s11102-018-0899-x. [DOI] [PubMed] [Google Scholar]
- 6.Hoorn EJ, Zietse R. Diagnosis and treatment of hyponatremia: Compilation of the guidelines. J Am Soc Nephrol. 2017;28(5):1340–49. doi: 10.1681/ASN.2016101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muhsin SA, Mount DB. Diagnosis and treatment of hypernatremia. Best Pract Res Clin Endocrinol Metab. 2016;30(2):189–203. doi: 10.1016/j.beem.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Gulsin GS, Jacobs MLB, Gohil S, et al. Competing interests in a lung cancer with metastasis to the pituitary gland: syndrome of inappropriate ADH secretion versus diabetes insipidus. Oxf Med Case Reports. 2016;2016(6):125–29. doi: 10.1093/omcr/omw044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiordoliva I, Meletani T, Baleani MG, et al. Managing hyponatremia in lung cancer: Latest evidence and clinical implications. Ther Adv Med Oncol. 2017;9(11):711–19. doi: 10.1177/1758834017736210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chabre O, Goichot B, Zenaty D, Bertherat J. Group 1. Epidemiology of primary and secondary adrenal insufficiency: prevalence and incidence, acute adrenal insufficiency, long-term morbidity and mortality. Ann Endocrinol. 2017;78(6):490–94. doi: 10.1016/j.ando.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Atallah-Yunes SA, Clark J, Samanani S, Soe M. Small cell lung cancer with pituitary metastasis presenting as secondary adrenal insufficiency: A case report and literature review. Am J Case Rep. 2019;20:207–11. doi: 10.12659/AJCR.913388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Non L, Brito D, Anastasopoulou C. Neurosarcoidosis-associated central diabetes insipidus masked by adrenal insufficiency. BMJ Case Rep. 2015;2015 doi: 10.1136/bcr-2014-206390. : bcr2014206390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carvalho F, Louro F, Zakout R. Adrenal Insufficiency in Metastatic Lung Cancer. World J Oncol. 2015;6(3):375–77. doi: 10.14740/wjon890w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin-Grace J, Dineen R, Sherlock M, Thompson CJ. Adrenal insufficiency: Physiology, clinical presentation and diagnostic challenges. Clinica Chim Acta. 2020;505:78–91. doi: 10.1016/j.cca.2020.01.029. [DOI] [PubMed] [Google Scholar]
- 15.Tallis PH, Rushworth RL, Torpy DJ, Falhammar H. Adrenal insufficiency due to bilateral adrenal metastases – a systematic review and meta-analysis. Heliyon. 2019;5(5):e01783. doi: 10.1016/j.heliyon.2019.e01783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowley RK, Argese N, Tomlinson JW, Stewart PM. Central hypoadrenalism. J Clin Endocrinol Metab. 2014;99(11):4027–36. doi: 10.1210/jc.2014-2476. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: Current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311. doi: 10.1016/S0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- 18.Kitajima K, Ashida K, Wada N, et al. Isolated ACTH deficiency probably induced by autoimmune-related mechanism evoked with nivolumab. Jpn J Clin Oncol. 2017;47(5):463–66. doi: 10.1093/jjco/hyx018. [DOI] [PubMed] [Google Scholar]
- 19.Yano S, Ashida K, Sakamoto R, et al. Human leucocyte antigen dr15, a possible predictive marker for immune checkpoint inhibitor-induced secondary adrenal insufficiency. Eur J Cancer. 2020;130:198–203. doi: 10.1016/j.ejca.2020.02.049. [DOI] [PubMed] [Google Scholar]