Extended Data Fig. 2. Automated spectrum assignment identifies PG modifications on C. burnetii OM proteins.
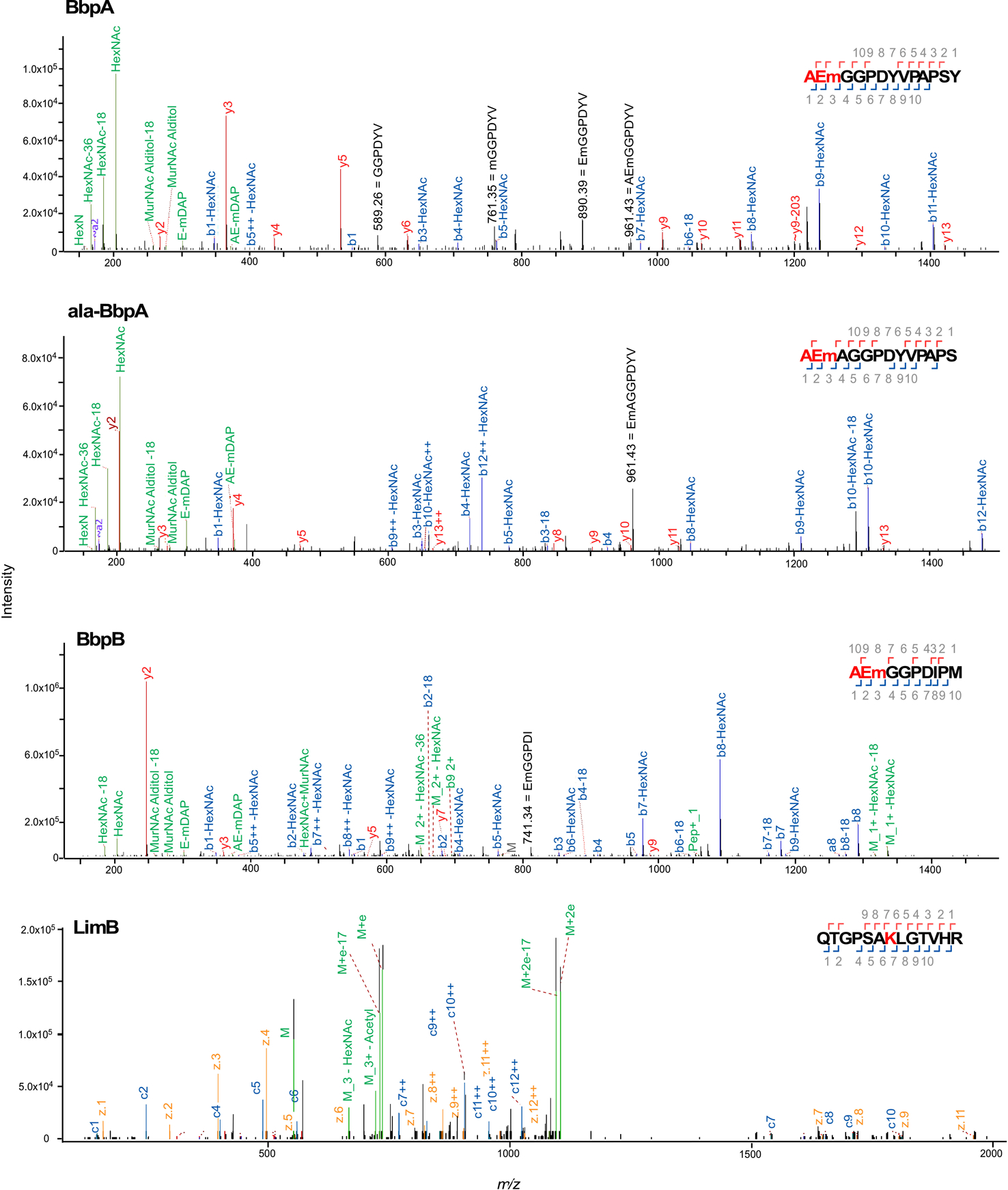
Representative MS/MS spectra for C. burnetii BbpA, ala-BbpA, BbpB β-barrel proteins and the lipoprotein LimB, covalently attached to mDAP (m) residues of PG. Spectra are shown as annotated by Byonic with manual annotations corresponding to internal fragments in black. J[+72.0848] has been replaced by m to represent mDAP. The PG tripeptide (AEm) is highlighted in red in the HCD spectra showing covalent attachment of PG to BbpA, ala-BbpA, and BbpB. The LimB Lys21 residue with PG tripeptide modification is highlighted in red in the ETD spectra showing covalent attachment of PG to LimB.
