Extended Data Fig. 7. β-barrels form a tight tether between the OM and PG.
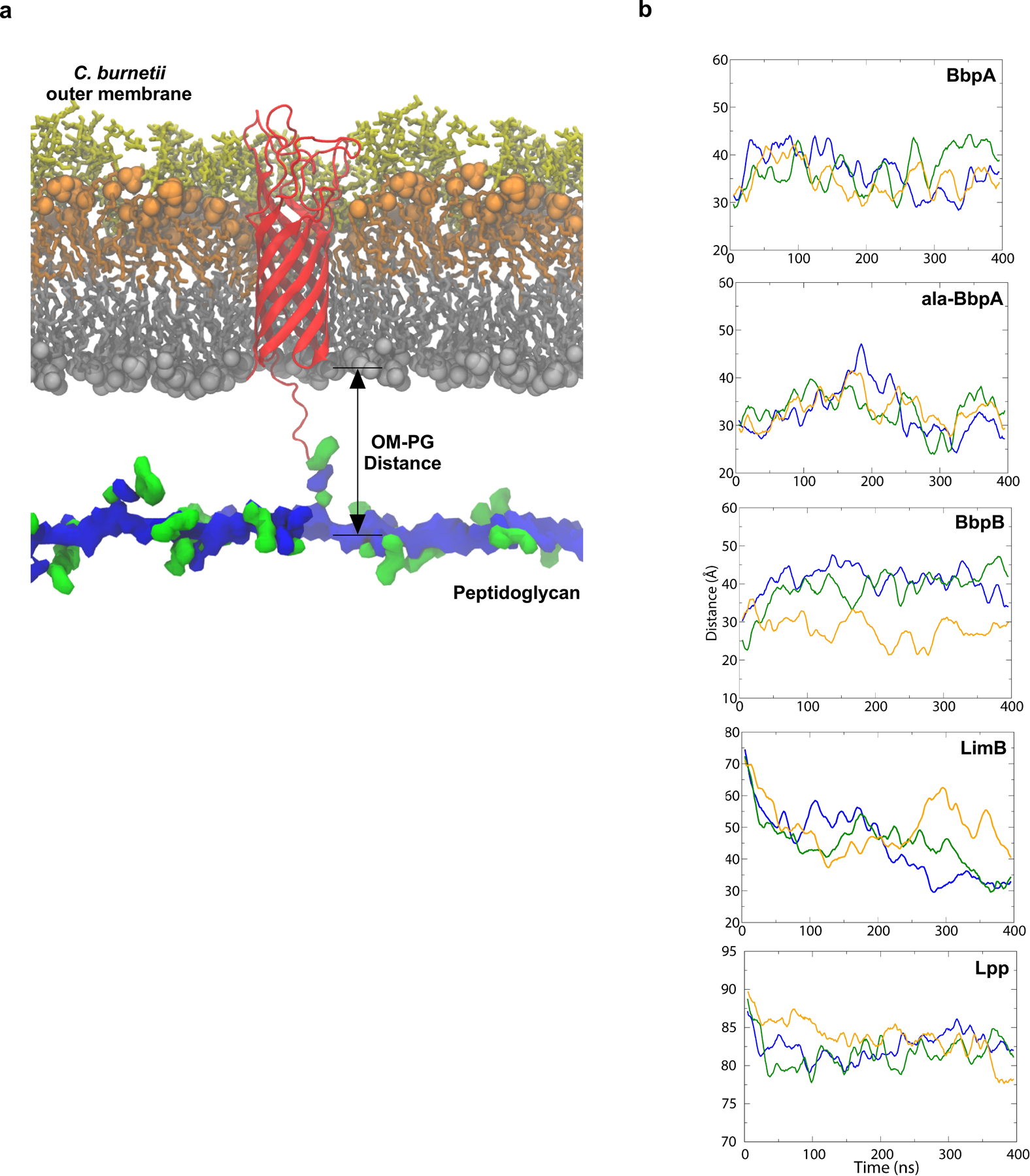
Structural model of BbpA (red) in the C. burnetii OM. Structures of the cell envelope are colored as follows: inner leaflet of OM (grey), lipid A of LPS (orange), core oligosaccharides (yellow), glycan chains of PG (blue) peptide stems of PG (green). A similar model was generated for BbpB. b. Molecular dynamics simulation of C. burnetti OM-PG protein-tethered models. The distance in angstroms between the phosphorus atoms of the inner leaflet of the OM and PG layers was measured for three runs for BbpA, ala-BbpA, BbpB, LimB, and Lpp from E. coli. The solid lines are running averages. Distances measured in angstroms for run 1, run 2, and run 3 are shown.
