Abstract
Glucocorticoids (GCs) are stress hormones that play multiple roles in the regulation of cancer cell differentiation, apoptosis, and proliferation. Some types of cancers, such as hematological malignancies, can be effectively treated by GCs, whereas the responses of epithelial cancers to GC treatment vary, even within cancer subtypes. In particular, GCs are frequently used as supporting treatment of breast cancer (BC) to protect against chemotherapy side effects. In the therapy of nonaggressive luminal subtypes of BC, GCs can have auxiliary antitumor effects due to their cytotoxic actions on cancer cells. However, GCs can promote BC progression, colonization of distant metastatic sites, and metastasis. The effects of GCs on cell proliferation vary with BC subtype and its molecular profile and are realized via the activation of glucocorticoid receptor (GR), a well-known transcriptional factor involved in the regulation of the expression of multiple genes, cell-cell adhesion, and cell migration and polarity. This review focuses on the roles of GC signaling in the adhesion, migration, and metastasis of BC cells. We discuss the molecular mechanisms of GC actions that lead to BC metastasis and propose alternative pharmacological uses of GCs for BC treatment.
Keywords: Breast cancer, glucocorticoid, glucocorticoid receptor, selective glucocorticoid receptor agonists (SEGRA), REDD1, breast cancer metastasis
Introduction
Breast cancer (BC) is the most common cancer diagnosed among women worldwide.1 Most BC-related deaths are caused not by the primary tumor but by metastases at distant sites.2 Breast cells become malignant after acquiring the capacities of uncontrolled epithelial proliferation and angiogenesis,3 invasion into surrounding tissues and metastasis to distant organs (lymph nodes, lungs, bones, and brain).4 Mechanisms underlying the elevated motility of BC cells and the formation of metastases are extensively discussed but remain incompletely understood. However, some molecular markers of different BC subtypes have been described as strong prognostic and predictive factors. For instance, cell-surface protein human epidermal growth factor receptor 2 (HER2), a transmembrane tyrosine kinase receptor, augments the metastatic potential of BC cells; HER2-expressing cells have greater ability to produce metastases than HER2-negative cells.4-6 In contrast, estrogen receptor (ER), progesterone receptor (PR), and HER2-negative BC, also known as triple-negative breast cancer (TNBC), is associated with significantly increased risk of disease progression and tumor cell dissemination.7,8
Glucocorticoids (GCs) are frequently administered as supporting therapy for palliative purposes during BC treatment.9 However, the effects of GCs in BC therapy could be varied and depend on cancer subtype. For example, it has been demonstrated that GCs suppress ER-positive BC tumor growth via increased estrogen sulfotransferase expression and decreased production of estradiol in malignant cells.10 At the same time, GCs have been shown to decrease cell adhesion and stimulate cell motility, resulting in an increased risk of metastasis in TNBC.10-16 Moreover, glucocorticoid receptor (GR) activation has been found to stimulate proliferation, enhance escape from apoptosis, and further disease progression.9,17 This review focuses on the roles of GC signaling in BC metastasis and the mechanisms underlying the effects of GCs on BC progression. In addition, possible alternatives of GC-based BC therapies are discussed.
Mechanisms of BC Metastasis
Metastasis is a multistage process that begins with the local invasion of cells from the primary tissue into the surrounding host tissue and continues until the tumor cells invade the bloodstream.18,19 Invasion begins with alterations of cell-cell adhesion and cell adhesion to the extracellular matrix (ECM). There are several types of connecting junctions: (1) focal adhesions, which are large protein complexes that interact with ECM via the binding of integrins with their ligands vinculin, collagen, laminin, phybronectin, and so on.20; (2) tight junctions, which occur as a series of very close membrane appositions of adjacent cells and are formed by transmembrane proteins such as occludin, claudins, junctional adhesion molecules (JAMs), and tricellulin and many scaffold proteins21; (3) adherens junctions, composed of complexes of transmembrane cadherins with cytoplasmic catenins and actin cytoskeleton21; (4) gap junctions, which are formed by the docking of 2 hemichannels (or connexons) on the cellular membrane composed of 6 transmembrane proteins called connexins22; and (5) desmosomes and hemidesmosomes, which are cadherin-based, multiprotein complexes, provide intercellular adhesion via connections of intermediate filaments.23 Focal, tight and gap junctions are the most affected by GC in BC.
The alterations in adhesive properties observed in BC cells include changes in the expression and activity of the majority of key adherence molecules, which can affect disease progression negatively or positively. Focal adhesion kinases stimulate cell motility and invasion in HER2-positive and ER-positive BC and in TNBC.24,25 However, BC cells lose their expression of tight junction proteins during malignant transformation.26 Connexin 43 is considered a potential tumor suppressor, as Wnt-dependent increases in its expression lead to the fortification of gap junctions27; however, connexins 26 and 30 have been shown to have oncogenic properties.28 The cadherin family has been documented to play a large role in mediating cell-cell adhesion and plays predominant roles in BC metastasis.29 The downregulation of E-cadherin and the upregulation of N-cadherin have been reported to reflect progression and metastasis in BC and to be associated with poor prognosis.19
Degradation of the ECM is an essential step before invasion and is carried out mainly through matrix metalloproteinases (MMPs) and the urokinase plasminogen activator (uPA) system. The expression of uPA is used as prognostic marker of distant metastases in BC patients.19,30
Epithelial-mesenchymal transition (EMT) is a critical pathway in the movement of tumor cells and a central driving force of cancer cell dissemination.19,29 The initial steps of EMT are the disintegration of cell-cell adhesion followed by an alteration in cell polarity from apical-basal to front-rear and activation of proteolytic enzymes such as MMPs.31 The EMT program in BC cells is associated with the upregulation of signaling pathways involved in tumor progression, such as the tumor growth factor (TGF)-β, Wnt, Notch, Hedgehog, estimated glomerular filtration rate (EGFR), platelet-derived growth factor receptor (PDGFR), and PI3K/AKT pathways, and poor prognosis.19 The mechanical force used is generated by active myosin/actin contractions and cortical actin via Rho small GTPase signaling.32,33
A suitable microenvironment is required to establish tumor growth and progression in both primary and metastatic sites. The establishment of such an environment involves specialized cells, including fibroblasts, immune cells, endothelial cells, and tissue-associated macrophages, which are capable of influencing tumor invasion, angiogenesis, immune evasion, and migratory behavior.19 Specific profiles of gene expression affect the target of BC metastasis (Table 1).
Table 1.
Organotropism of BC metastasis.
| Target organ | BC subtype | Associated molecules/pathways |
|---|---|---|
| Lung | TNBC, Luminal B | MMP1a, MMP2, ID1, CXCL1, EREG, COX-2, TGF-beta, LOX |
| Brain | TNBC, Luminal B, HER2+ | ST6GALNAC5, HBEGF, COX-2, IL8, L1CAM |
| Liver | Luminal A and B, HER2+ | E-selectine, CXCR4, CD44, E-, N-cadherins |
| Bone | Luminal B | PTHRP, RANKL, SHH, ITGAV, NOTCH, TGF-beta, VCAM-1 |
Abbreviations: BC, breast cancer; CD44, CD44 molecule (Indian blood group); COX2, cyclooxygenase 2; CXCL1, chemokine (C-X-C motif) ligand 1; CXCR4, C-X-C motif chemokine receptor 4; EREG, epiregulin; HBEGF, heparin-binding epidermal growth factor; HER2, human epidermal growth factor receptor 2; ID1, inhibitor of DNA binding 1; IL8, interleukin 8; ITGAV, integrin subunit alpha V; L1CAM, L1 cell adhesion molecule; LOX, lysyl oxidase; MMP, matrix metalloproteinase; PTHRP, parathyroid hormone like hormone; RANKL, TNF superfamily member 11; SHH, sonic hedgehog signaling molecule; ST6GALNAC5ST6, N-acetylgalactosaminide alpha-2,6-sialyltransferase 5; TGF beta, tumor growth factor beta; TNBC, triple negative breast cancer; VCAM, vascular cell adhesion molecule 1.
Genes in bold have strong association with GR activity.
Glucocorticoids in BC Metastasis
Glucocorticoids are administered in various doses before, during, and after chemotherapy in BC patients because of their benefits in improving patient quality of life and their antiemetic effects.9,34,35 However, the use of GCs in BC treatment continues to be debated. It has been reported in multiple studies that GCs can suppress tumor progression and metastasis,34,36 but other studies have demonstrated the ability of GCs to protect cancer cells from apoptosis and to diminish the cytotoxic effects of main chemotherapeutics.37,38 The role of the GR in BC biology appears to be dependent on ER expression and activity,37 suggesting that it likely varies between BC subtypes. A high expression level of GR correlates with good prognosis in ER-positive BC and poor prognosis in TNBC.14,39,40 Furthermore, it has been shown in several in vitro models of basal-like BC that GCs suppress cell migration and invasion by inhibiting the expression of RhoA or by inducing E-cadherin expression.41 In contrast, another study demonstrated that in both in vitro and in vivo models of TNBC, dexamethasone increased the metastatic potential of BC cells, possibly via the enhanced expression of receptor tyrosine kinase (RTK)-like orphan receptor 1 (ROR1), which is associated with increased colonization of metastatic niches, aggressive disease, and poor outcome.14,42
Mechanism of GR action and its effect on cell motility and adhesion
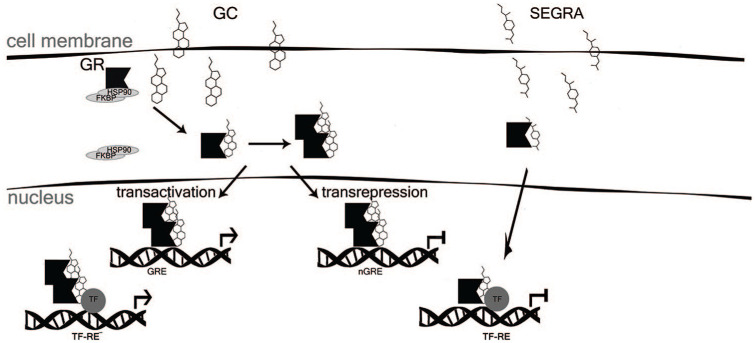
Glucocorticoids exert their biological effects by binding to the intracellular GR, a well-known transcription factor expressed in normal, preinvasive, and invasive cancers.43 Upon hormone binding, GR translocates to the nucleus, where it regulates gene expression by either (1) transactivation (TA) via GR homodimer binding to GC-responsive elements (GRE) or (2) transrepression (TR), which is frequently mediated by negative interactions between GR and other TFs, including proinflammatory nuclear factor kappa beta (NF-κB)44-47 (Figure 1).
Figure 1.
Mechanism of GR activation by GC and by SEGRA. FKBP indicates FK506 binding protein; GC, glucocorticoids; GR, glucocorticoid receptor; GRE, glucocorticoid responsive element; HSP, heat shock protein; nGRE, negative GRE; SEGRA, selective glucocorticoid receptor agonists; TF, transcription factor; TF-RE, complex of TF with responsive element.
The anti-inflammatory effects of GCs are mediated via GR TR, whereas the undesirable side effects (insulin resistance, skin atrophy, osteoporosis, and GC resistance) are thought to occur mainly via the activation of gene transcription (GR TA).44 Since some genes closely related to BC metastasis (ILs, COX-2, TNFα) are involved in inflammation, GCs can simultaneously affect metastasis and inflammation.
The nongenomic effects of GCs on cell junctions in BC are poorly understood. It has been shown in primary keratinocytes, that GR localizes on plasma membrane in complex with a-catenin,48 but the exact mechanism remains unknown. The genomic mechanisms underlying the effects of GR involve the expression regulation either via TR or TA of multiple adhesion molecules, including occludins, claudins, E-cadherin, β-catenin, plakoglobin,49 and proteins from the Rho family.40 GR effects on the cell-cell adhesion of BC cells vary depending on cell subtype and microenvironment conditions. Glucocorticoid-receptor-dependent restoration of claudin-1 expression was observed in three-dimensional BC spheroids but not in cell monolayer.50 Glucocorticoids induce the expression of key genes of tight contacts in tumor cells as well as in the tissue microenvironment. Glucocorticoids can modulate the properties of the vascular endothelial barrier via increased occludin and claudin-5 expression.51 In rat BC cell line Con8, GC was found to stimulate the formation of tight and adherens junctions via the inhibition of small GTPase RhoA.40 In a zebrafish model, it was shown that the GC prednisolone contributes to the downregulation of itga10 and itgbl1 genes, which are involved in collagen binding and cell-matrix adhesion.52 These findings are consistent with the observation that a GC-induced decrease of BC metastatic potential was associated with E- or N-cadherin transition from the cytoplasmic to transmembrane localization as well as increased expression of these genes. Enhanced expression of GC-regulated IL-6 in cells of the ER-positive ZR-75-1 and T-47D cells, derived from ductal carcinoma, induced a shift to the fibroblastoid morphology, enhanced motility, increased cell-cell separation, and decreased the stability of adherens-type junctions.53 Another mechanism of stabilization of the E-cadherin-β-catenin complex by GC is the downregulation of the actin-binding protein fascin, a negative regulator of adherens junctions.54 Glucocorticoid effects on gap junction formation have been described mainly for nontumor tissues and are debated. For example, the expression of connexins 26 and 32 in primary hepatocytes was found to be increased after GC treatment in rat model of pancreas adenocarcinoma, whereas the expression of connexin 30 has been shown to be decreased following GC treatment in gliomas and healthy brain tissue as well as in osteoblasts.55,56 However, effects of GCs on gap junctions in BC cells remain unknown.
Moreover, the GR gene produces multiple transcriptional and translational GR isoforms. Fully functioning GRα protein is encoded by 9 exons in the GR gene; other GR isoforms lack some functions and could be another reason for GC resistance.57 A study of cell junction formation and functioning, it was demonstrated that GRβ, which lacks the ligand-binding domain for GCs and has been shown to be inhibitory to GRα, increases the migration of human bladder cancer cells.58 A diversity of GR isoforms is associated with alternative translation initiation, as multiple AUG start codons exist in the GRα mRNA sequence.57 These GRα isoforms have distinct cellular localization and gene regulatory profiles.57 Additional posttranslational modifications of GR include ubiquitination, acetylation, and SUMOylation. These modifications can alter receptor activity and enhance the functional diversity of the receptors and thus profoundly impact subsequent signaling. For example, GRs are modified by acetylation at Lys-494 and Lys-495 in response to GCs. This modification impairs the ability of GR to interfere with NF-κB signaling.59 Since some NF-kB-dependent genes are closely related to BC metastasis, this posttranslation modification of GR can affect BC cell migration and invasion, but the exact mechanisms remain unknown.
Effect of GR on EMT
The consequences of cross-talk between GR and the signaling pathways involved in EMT in BC vary depending on the molecular subtype of disease (Table 2). For example, it has been shown in the GFP-ERa:PRL-HeLa array cell line, consisting of multicopy integration of an estrogen-responsive transcriptional reporter gene and also stably expresses GFP-ERa (green fluorescent protein-tagged) at levels comparable with MCF-7 BC cells, that GR binds to ER promoters, displacing ER and repressing proliferative activity.60 These effects depend on both ER and GR function and may have important implications for GC influence on the global ER program and specifically on EMT in ER-positive cancers. Regarding ER-negative cancers, dysfunctional GR signaling has been described in tumors with BRCA mutations,15 and EMT promotion in TNBC cells with BRCA1 dysfunction may be independent of GR. Nevertheless, EMT was also identified as one of the primary processes regulated by GR in a ChIP-seq study of ER-negative cells.39 In addition, annotated transcriptome studies have shown that the prognostic information of GR expression differs between ER-positive and ER-negative patients. In ER-negative BC patients, upregulated GR results in increased activity of multiple members of the Wnt and Hippo pathways and is associated with worse prognosis, whereas in ER-positive BC patients, high levels of GR expression in tumors are significantly associated with better outcome.14,15 Consistent with these observations, it was reported that GR-induced tumor invasion, EMT, and lung metastasis in vivo. Glucocorticoid-receptor-mediated suppression of insulin receptor substrate-1 followed by activation of the ERK2 MAP kinase pathway was shown to play the key role in GR-induced EMT.61 However, GR has been found to attenuate invasion of ER-negative BC cells via the induction of CCN5/WISP-2 expression and transcriptional repression of EMT-associated genes, in particular, the mesenchymal marker vimentin and the mesenchymal transcription factor ZEB1.62
Table 2.
Differences of GCs functions in ER+ and ER-BC.
| BC subtype | GC effects | Result |
|---|---|---|
| Luminal (ER+) | TR of AKT/mTOR, CCND1, CDK2, CDK6 | Block the cytoskeleton organizations and cell migrations, inhibition of proliferation |
| Basal (TNBC) |
TA of ROR1, CDK1, MKP1, SGK1, PDK4, TSC22D, CCN5/WISP-2 and KLF9. TR of Bid, CD95 L, TRAIL, Bcl-xL, IRS-1, PRAG1, PLK2, CDH11, HGF, ZNF703 and SOX9 |
Increase survival, proliferation, metastatic potential, tamoxifen resistance |
Abbreviations: BC, breast cancer; Bcl-xL, BCL2-like 1; Bid, BH3 interacting domain death agonist; CCND1, cyclin D1; CCN5/WISP-2, cellular communication network factor 5; CDH11, cadherin 11; CDK1, cyclin-dependent kinase 1; CDK2, cyclin-dependent kinase 2; CDK6, cyclin-dependent kinase 6; CD95L, Fas ligand; ER, estrogen receptor; GC, glucocorticoid; HGF, hepatocyte growth factor; IRS1, insulin receptor substrate 1; KLF9, Kruppel-like factor 9; MKP1, mitogen-activated protein kinase phosphatase 1; mTOR, mammalian target of rapamycin; PDK4, pyruvate dehydrogenase kinase 4; PLK2, polo-like kinase 2; PRAG1, PEAK1-related, kinase-activating pseudokinase 1; ROR1, receptor-tyrosine-kinase-like orphan receptor 1; SGK1, serum/glucocorticoid-regulated kinase 1; SOX9, SRY-box transcription factor 9; TA, transactivation; TNBC, triple-negative breast cancer; TR, transrepression; TRAIL, TNF superfamily member 10; TSC22D, TSC22 domain family, member 3; ZNF703, zinc finger protein 703.
Selective Activation of GR in BC Metastasis
It is well accepted that GR TR plays an important role in the anti-inflammatory effects of GCs. However, many side effects of steroids (glucose metabolism, steroid diabetes, osteoporosis, skin and muscle atrophy) strongly depend on GR TA.44,47 Although some concepts in the GR field have been revised, it remains well accepted that selective glucocorticoid receptor agonists (SEGRAs) that shift GR activity toward TR have a better therapeutic index than classical glucocorticoids.47,63-67 Selective glucocorticoid receptor agonists that do not support GR dimerization and GR-mediated TA have better therapeutic index than classical GC.44,47 The therapeutic anti-inflammatory and anticancer efficacy of different SEGRAs has been confirmed in multiple studies.44,47,68,69 However, data on the possible implications of SEGRAs in the treatment of metastatic BC are insufficient. It has been shown that a SEGRA of natural origin, CpdA, effectively inhibited the growth and proliferation of MDA-MB-231 and MCF7 cells, did not promote drug resistance and did not attenuate the cytotoxicity of the main therapeutics.68,69 Similar observations were described for another SEGRA, rigid steroid 21-hydroxy-6,19-epoxyprogesterone.70 However, no data are yet available on the specific SEGRA effects on key components of cell motility, migration, EMT, and metastatic potential.
An alternative approach yielding safer GR-targeted therapies could be the combination of GCs with compounds that can protect tissues against GC side effects. Recently, we identified REDD1 (regulated in development and DNA damage response 1), a negative regulator of mTOR/Akt signaling and a marker of cellular stresses, including hypoxia, DNA damage and GCs,71 as a key regulator of the development of atrophic complications in skin.72 In studies by ourselves and others, REDD1 was strongly induced during GC-dependent atrophy in skin and muscle, and REDD1-knockout animals were protected against steroid-induced skin atrophy and muscle waste.72,73 Moreover, the lack of REDD1 did not affect the anti-inflammatory effects of GC.72 We demonstrated that the compounds from the PI3K/Akt/mTOR modulator class inhibited GC-induced REDD1 expression and strongly attenuated GR TA along with GR TR acceleration in keratinocytes and transformed lymphocytes. However, the effects of the inhibition of GC-induced REDD1 expression have not yet been studied in BC cells and require investigation. Nevertheless, a combination of GC with REDD1 inhibitors could potentially ameliorate some of the metabolic adverse effects of GС, suggesting clinical applications for a wide range of diseases and disorders treated with GС.74 Little is known about the role of REDD1 in the regulation of the proliferation of BC cells, and the data to date have yielded mixed results. For example, several studies have shown that an increase of REDD1 expression after treatment with different chemotherapeutics is associated with a decreased viability of BC cells.75,76 Furthermore, in HER2-positive BC and in TNBC, tumor cell proliferation and survival in the hypoxic tumor environment might be promoted by disinhibition of the mTOR pathway and HIF-1α stabilization by the downregulation of REDD1.77 On the contrary, Pinto and colleagues demonstrated that REDD1 expression was associated with poor outcome in TNBC.78 Evidently, even selective activation of GR can lead to different outcomes in different molecular subtypes of BC, which indicates that the molecular interactions that underlie the relationships between GR and the processes in BC are complex.
Conclusion
Overall, the roles of GR signaling in cell adhesion, EMT, invasion, and metastasis in BC remain unclear. The effects of GC depend on BC subtype, tumor microenvironment, and GR transcriptional activity. Selective glucocorticoid receptor agonists might provide the option to shift GR function toward antimetastatic activity.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Russian Science Foundation (grant no. 17-75-20124-П to E.A.L.).
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: EMZ, EAL, KIK, MGY, GAB drafted the manuscript. AVS, SAM contributed to writing and editing the manuscript. All authors read and approved the final manuscript.
ORCID iDs: Ekaterina M Zhidkova  https://orcid.org/0000-0003-3318-9391
https://orcid.org/0000-0003-3318-9391
Evgeniya S Lylova  https://orcid.org/0000-0001-6388-1624
https://orcid.org/0000-0001-6388-1624
Kirill I Kirsanov  https://orcid.org/0000-0002-8599-6833
https://orcid.org/0000-0002-8599-6833
Ekaterina A Lesovaya  https://orcid.org/0000-0002-1967-9637
https://orcid.org/0000-0002-1967-9637
References
- 1. DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61:409-418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 2. Gunasinghe NPAD, Wells A, Thompson EW, Hugo HJ. Mesenchymal–epithelial transition (MET) as a mechanism for metastatic colonisation in breast cancer. Cancer Metastasis Rev. 2012;31:469-478. doi: 10.1007/s10555-012-9377-5. [DOI] [PubMed] [Google Scholar]
- 3. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57-70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4. Sharma R, Sharma R, Khaket TP, Dutta C, Chakraborty B, Mukherjee TK. Breast cancer metastasis: putative therapeutic role of vascular cell adhesion molecule-1. Cell Oncol. 2017;40:199-208. doi: 10.1007/s13402-017-0324-x. [DOI] [PubMed] [Google Scholar]
- 5. Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271-3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 6. Murthy P, Kidwell KM, Schott AF, et al. Clinical predictors of long-term survival in HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2016;155:589-595. doi: 10.1007/s10549-016-3705-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kozłowski J, Kozłowska A, Kocki J. Breast cancer metastasis—insight into selected molecular mechanisms of the phenomenon. Postepy Hig Med Dosw. 2015;69:447-451. doi: 10.5604/17322693.1148710. [DOI] [PubMed] [Google Scholar]
- 8. Lorusso G, Rüegg C. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem Cell Biol. 2008;130:1091-1103. doi: 10.1007/s00418-008-0530-8. [DOI] [PubMed] [Google Scholar]
- 9. Volden PA, Conzen SD. The influence of glucocorticoid signaling on tumor progression. Brain Behav Immun. 2013;30:S26-S31. doi: 10.1016/j.bbi.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gong H, Jarzynka MJ, Cole TJ, et al. Glucocorticoids antagonize estrogens by glucocorticoid receptor-mediated activation of estrogen sulfotransferase. Cancer Res. 2008;68:7386-7393. doi: 10.1158/0008-5472.CAN-08-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fakih M, Johnson CS, Trump DL. Glucocorticoids and treatment of prostate cancer: a preclinical and clinical review. Urology. 2002;60:553-561. http://www.ncbi.nlm.nih.gov/pubmed/12385906. [DOI] [PubMed] [Google Scholar]
- 12. Zhang C, Wenger T, Mattern J, et al. Clinical and mechanistic aspects of glucocorticoid-induced chemotherapy resistance in the majority of solid tumors. Cancer Biol Ther. 2007;6:278-287. doi: 10.4161/cbt.6.2.3652. [DOI] [PubMed] [Google Scholar]
- 13. McNamara KM, Kannai A, Sasano H. Possible roles for glucocorticoid signalling in breast cancer. Mol Cell Endocrinol. 2018;466:38-50. doi: 10.1016/j.mce.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 14. Obradović MMS, Hamelin B, Manevski N, et al. Glucocorticoids promote breast cancer metastasis. Nature. 2019;567:540-544. doi: 10.1038/s41586-019-1019-4. [DOI] [PubMed] [Google Scholar]
- 15. Veneris JT, Huang L, Churpek JE, Conzen SD, Fleming GF. Glucocorticoid receptor expression is associated with inferior overall survival independent of BRCA mutation status in ovarian cancer. Int J Gynecol Cancer. 2019;29:357-364. doi: 10.1136/ijgc-2018-000101. [DOI] [PubMed] [Google Scholar]
- 16. Sasson R, Amsterdam A. Pleiotropic anti-apoptotic activity of glucocorticoids in ovarian follicular cells. Biochem Pharmacol. 2003;66:1393-1401. doi: 10.1016/s0006-2952(03)00489-1. [DOI] [PubMed] [Google Scholar]
- 17. Belova L, Delgado B, Kocherginsky M, Melhem A, Olopade OI, Conzen SD. Glucocorticoid receptor expression in breast cancer associates with older patient age. Breast Cancer Res Treat. 2009;116:441-447. doi: 10.1007/s10549-008-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hunter K, Welch DR, Liu ET. Genetic background is an important determinant of metastatic potential. Nat Genet. 2003;34:23-24. doi: 10.1038/ng0503-23b. [DOI] [PubMed] [Google Scholar]
- 19. Scully OJ, Bay B-H, Yip G, Yu Y. Breast cancer metastasis. Cancer Genomics Proteomics. 2012;9:311-320. http://www.ncbi.nlm.nih.gov/pubmed/22990110. [PubMed] [Google Scholar]
- 20. Maziveyi M, Alahari SK. Cell matrix adhesions in cancer: the proteins that form the glue. Oncotarget. 2017;8:48471-48487. doi: 10.18632/oncotarget.17265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dejana E, Orsenigo F. Endothelial adherens junctions at a glance. J Cell Sci. 2013;126:2545-2549. doi: 10.1242/jcs.124529. [DOI] [PubMed] [Google Scholar]
- 22. Mao X-Y, Li Q-Q, Gao Y-F, Zhou H-H, Liu Z-Q, Jin W-L. Gap junction as an intercellular glue: emerging roles in cancer EMT and metastasis. Cancer Lett. 2016;381:133-137. doi: 10.1016/j.canlet.2016.07.037. [DOI] [PubMed] [Google Scholar]
- 23. Rübsam M, Broussard JA, Wickström SA, Nekrasova O, Green KJ, Niessen CM. Adherens junctions and desmosomes coordinate mechanics and signaling to orchestrate tissue morphogenesis and function: an evolutionary perspective. Cold Spring Harb Perspect Biol. 2018;10:a029207. doi: 10.1101/cshperspect.a029207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sanchez AM, Flamini MI, Baldacci C, Goglia L, Genazzani AR, Simoncini T. Estrogen receptor-α promotes breast cancer cell motility and invasion via focal adhesion kinase and N-WASP. Mol Endocrinol. 2010;24:2114-2125. doi: 10.1210/me.2010-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gari HH, DeGala GD, Ray R, Lucia MS, Lambert JR. PRL-3 engages the focal adhesion pathway in triple-negative breast cancer cells to alter actin structure and substrate adhesion properties critical for cell migration and invasion. Cancer Lett. 2016;380:505-512. doi: 10.1016/j.canlet.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kobayashi K, Tsugami Y, Matsunaga K, Oyama S, Kuki C, Kumura H. Prolactin and glucocorticoid signaling induces lactation-specific tight junctions concurrent with β-casein expression in mammary epithelial cells. Biochim Biophys Acta. 2016;1863:2006-2016. doi: 10.1016/j.bbamcr.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 27. Fostok S, El-Sibai M, Bazzoun D, Lelièvre S, Talhouk R. Connexin 43 loss triggers cell cycle entry and invasion in non-neoplastic breast epithelium: a role for noncanonical Wnt signaling. Cancers. 2019;11:339. doi: 10.3390/cancers11030339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Teleki I, Szasz AM, Maros ME, et al. Correlations of differentially expressed gap junction connexins Cx26, Cx30, Cx32, Cx43 and Cx46 with breast cancer progression and prognosis. PLoS ONE. 2014;9:e112541. doi: 10.1371/journal.pone.0112541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gloushankova NA, Rubtsova SN, Zhitnyak IY. Cadherin-mediated cell-cell interactions in normal and cancer cells. Tissue Barriers. 2017;5:e1356900. doi: 10.1080/21688370.2017.1356900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harbeck N, Kates RE, Schmitt M, et al. Urokinase-type plasminogen activator and its inhibitor type 1 predict disease outcome and therapy response in primary breast cancer. Clin Breast Cancer. 2004;5:348-352. doi: 10.3816/cbc.2004.n.040. [DOI] [PubMed] [Google Scholar]
- 31. Iwatsuki M, Mimori K, Yokobori T, et al. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010;101:293-299. doi: 10.1111/j.1349-7006.2009.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yilmaz M, Christofori G. Mechanisms of motility in metastasizing cells. Mol Cancer Res. 2010;8:629-642. doi: 10.1158/1541-7786.MCR-10-0139. [DOI] [PubMed] [Google Scholar]
- 33. Friedl P, Wolf K. Tube travel: the role of proteases in individual and collective cancer cell invasion. Cancer Res. 2008;68:7247-7249. doi: 10.1158/0008-5472.CAN-08-0784. [DOI] [PubMed] [Google Scholar]
- 34. Keith BD. Systematic review of the clinical effect of glucocorticoids on nonhematologic malignancy. BMC Cancer. 2008;8:84. doi: 10.1186/1471-2407-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vilasco M, Communal L, Mourra N, Courtin A, Forgez P, Gompel A. Glucocorticoid receptor and breast cancer. Breast Cancer Res Treat. 2011;130:1-10. doi: 10.1007/s10549-011-1689-6. [DOI] [PubMed] [Google Scholar]
- 36. Mikosz CA, Brickley DR, Sharkey MS, Moran TW, Conzen SD. Glucocorticoid receptor-mediated protection from apoptosis is associated with induction of the serine/threonine survival kinase gene, sgk-1. J Biol Chem. 2001;276:16649-16654. doi: 10.1074/jbc.M010842200. [DOI] [PubMed] [Google Scholar]
- 37. Kach J, Conzen SD, Szmulewitz RZ. Targeting the glucocorticoid receptor in breast and prostate cancers. Sci Transl Med. 2015;7:305ps19. doi: 10.1126/scitranslmed.aac7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Y, Zhou D, Phung S, Masri S, Smith D, Chen S. SGK3 is an estrogen-inducible kinase promoting estrogen-mediated survival of breast cancer cells. Mol Endocrinol. 2011;25:72-82. doi: 10.1210/me.2010-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pan D, Kocherginsky M, Conzen SD. Activation of the glucocorticoid receptor is associated with poor prognosis in estrogen receptor-negative breast cancer. Cancer Res. 2011;71:6360-6370. doi: 10.1158/0008-5472.CAN-11-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rubenstein NM, Callahan JA, Lo DH, Firestone GL. Selective glucocorticoid control of Rho kinase isoforms regulate cell–cell interactions. Biochem Biophys Res Commun. 2007;354:603-607. doi: 10.1016/j.bbrc.2007.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Law ME, Corsino PE, Jahn SC, et al. Glucocorticoids and histone deacetylase inhibitors cooperate to block the invasiveness of basal-like breast cancer cells through novel mechanisms. Oncogene. 2013;32:1316-1329. doi: 10.1038/onc.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chien H-P, Ueng S-H, Chen S-C, et al. Expression of ROR1 has prognostic significance in triple negative breast cancer. Virchows Arch. 2016;468:589-595. doi: 10.1007/s00428-016-1911-3. [DOI] [PubMed] [Google Scholar]
- 43. Buxant F, Engohan-Aloghe C, Noël J-C. Estrogen receptor, progesterone receptor, and glucocorticoid receptor expression in normal breast tissue, breast in situ carcinoma, and invasive breast cancer. Appl Immunohistochem Mol Morphol. 2010;18:254-257. doi: 10.1097/PAI.0b013e3181c10180. [DOI] [PubMed] [Google Scholar]
- 44. De Bosscher K, Beck IM, Dejager L, et al. Selective modulation of the glucocorticoid receptor can distinguish between transrepression of NF-κB and AP-1. Cell Mol Life Sci. 2014;71:143-163. doi: 10.1007/s00018-013-1367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ramamoorthy S, Cidlowski JA. Ligand-induced repression of the glucocorticoid receptor gene is mediated by an NCoR1 repression complex formed by long-range chromatin interactions with intragenic glucocorticoid response elements. Mol Cell Biol. 2013;33:1711-1722. doi: 10.1128/MCB.01151-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ratman D, Vanden Berghe W, Dejager L, et al. How glucocorticoid receptors modulate the activity of other transcription factors: a scope beyond tethering. Mol Cell Endocrinol. 2013;380:41-54. doi: 10.1016/j.mce.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 47. Lesovaya E, Yemelyanov A, Swart AC, Swart P, Haegeman G, Budunova I. Discovery of compound A: a selective activator of the glucocorticoid receptor with anti-inflammatory and anti-cancer activity. Oncotarget. 2015;6:30730-30744. doi: 10.18632/oncotarget.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stojadinovic O, Sawaya A, Pastar I, Tomic-Canic M. Glucocorticoid receptor localizes to adherens junctions at the plasma membrane of keratinocytes. PLoS ONE. 2013;8:e63453. doi: 10.1371/journal.pone.0063453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Firestone GL, Kapadia BJ. Minireview: steroid/nuclear receptor-regulated dynamics of occluding and anchoring junctions. Mol Endocrinol. 2014;28:1769-1784. doi: 10.1210/me.2014-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hoevel T, Macek R, Swisshelm K, Kubbies M. Reexpression of the TJ protein CLDN1 induces apoptosis in breast tumor spheroids. Int J Cancer. 2004;108:374-383. doi: 10.1002/ijc.11571. [DOI] [PubMed] [Google Scholar]
- 51. Felinski EA, Cox AE, Phillips BE, Antonetti DA. Glucocorticoids induce transactivation of tight junction genes occludin and claudin-5 in retinal endothelial cells via a novel cis-element. Exp Eye Res. 2008;86:867-878. doi: 10.1016/j.exer.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huo L, Wang L, Yang Z, Li P, Geng D, Xu Y. Prednisolone induces osteoporosis-like phenotypes via focal adhesion signaling pathway in zebrafish larvae. Biol Open. 2018;7:bio029405. doi: 10.1242/bio.029405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Krueger J, Ray A, Tamm I, Sehgal PB. Expression and function of interleukin-6 in epithelial cells. J Cell Biochem. 1991;45:327-334. doi: 10.1002/jcb.240450404. [DOI] [PubMed] [Google Scholar]
- 54. Wong V, Ching D, McCrea PD, Firestone GL. Glucocorticoid down-regulation of fascin protein expression is required for the steroid-induced formation of tight junctions and cell-cell interactions in rat mammary epithelial tumor cells. J Biol Chem. 1999;274:5443-5453. doi: 10.1074/jbc.274.9.5443. [DOI] [PubMed] [Google Scholar]
- 55. Hinkerohe D, Wolfkühler D, Haghikia A, Meier C, Faustmann PM, Schlegel U. Dexamethasone differentially regulates functional membrane properties in glioma cell lines and primary astrocytes in vitro. J Neurooncol. 2011;103:479-489. doi: 10.1007/s11060-010-0456-6. [DOI] [PubMed] [Google Scholar]
- 56. Swarovsky B, Steinhilber W, Scheele GA, Kern HF. Coupled induction of exocrine proteins and intracellular compartments involved in the secretory pathway in AR4-2J cells by glucocorticoids. Eur J Cell Biol. 1988;47:101-111. http://www.ncbi.nlm.nih.gov/pubmed/2465895. [PubMed] [Google Scholar]
- 57. Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132:1033-1044. doi: 10.1016/j.jaci.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McBeth L, Nwaneri AC, Grabnar M, Demeter J, Nestor-Kalinoski A, Hinds TD. Glucocorticoid receptor beta increases migration of human bladder cancer cells. Oncotarget. 2016;7:27313-27324. doi: 10.18632/oncotarget.8430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ito K, Yamamura S, Essilfie-Quaye S, et al. Histone deacetylase 2–mediated deacetylation of the glucocorticoid receptor enables NF-κB suppression. J Exp Med. 2006;203:7-13. doi: 10.1084/jem.20050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bolt MJ, Stossi F, Newberg JY, Orjalo A, Johansson HE, Mancini MA. Coactivators enable glucocorticoid receptor recruitment to fine-tune estrogen receptor transcriptional responses. Nucleic Acids Res. 2013;41:4036-4048. doi: 10.1093/nar/gkt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shi W, Wang D, Yuan X, et al. Glucocorticoid receptor–IRS-1 axis controls EMT and the metastasis of breast cancers. J Mol Cell Biol. 2019;11:1042-1055. doi: 10.1093/jmcb/mjz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ferrand N, Stragier E, Redeuilh G, Sabbah M. Glucocorticoids induce CCN5/WISP-2 expression and attenuate invasion in oestrogen receptor-negative human breast cancer cells. Biochem J. 2012;447:71-79. doi: 10.1042/BJ20120311. [DOI] [PubMed] [Google Scholar]
- 63. Sundahl N, Clarisse D, Bracke M, Offner F, Berghe W, Beck IM. Selective glucocorticoid receptor-activating adjuvant therapy in cancer treatments. Oncoscience. 2016;3:188-202. doi: 10.18632/oncoscience.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Meijer OC, Koorneef LL, Kroon J. Glucocorticoid receptor modulators. Ann Endocrinol. 2018;79:107-111. doi: 10.1016/j.ando.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 65. Safy M, de Hair MJH, Jacobs JWG, Buttgereit F, Kraan MC, van Laar JM. Efficacy and safety of selective glucocorticoid receptor modulators in comparison to glucocorticoids in arthritis, a systematic review. PLoS ONE. 2017;12:e0188810. doi: 10.1371/journal.pone.0188810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. De Bosscher K. Selective glucocorticoid receptor modulators. J Steroid Biochem Mol Biol. 2010;120:96-104. doi: 10.1016/j.jsbmb.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 67. Hua G, Zein N, Daubeuf F, Chambon P. Glucocorticoid receptor modulators CpdX and CpdX-D3 exhibit the same in vivo antiinflammatory activities as synthetic glucocorticoids. Proc Natl Acad Sci USA. 2019;116:14191-14199. doi: 10.1073/pnas.1908258116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen Z, Lan X, Wu D, et al. Ligand-dependent genomic function of glucocorticoid receptor in triple-negative breast cancer. Nat Commun. 2015;6:8323. doi: 10.1038/ncomms9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhidkova EM, Kuzin KA, Tilova LR, et al. Comparative analysis of biological effects of selective activator of the glucocorticoid receptor CpdA on different subtypes of breast cancer cell lines. Sib J Oncol. 2017;16:41-46. doi: 10.21294/1814-4861-2017-16-6-41-46. [DOI] [Google Scholar]
- 70. Orqueda AJ, Dansey MV, Español A, et al. The rigid steroid 21-hydroxy-6,19-epoxyprogesterone (21OH-6,19OP) is a dissociated glucocorticoid receptor modulator potentially useful as a novel coadjuvant in breast cancer chemotherapy. Biochem Pharmacol. 2014;89:526-535. doi: 10.1016/j.bcp.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 71. Dennis MD, Coleman CS, Berg A, Jefferson LS, Kimball SR. REDD1 enhances protein phosphatase 2A-mediated dephosphorylation of Akt to repress mTORC1 signaling. Sci Signal. 2014;7:ra68. doi: 10.1126/scisignal.2005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Baida G, Bhalla P, Kirsanov K, et al. REDD1 functions at the crossroads between the therapeutic and adverse effects of topical glucocorticoids. EMBO Mol Med. 2015;7:42-58. doi: 10.15252/emmm.201404601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Britto FA, Begue G, Rossano B, et al. REDD1 deletion prevents dexamethasone-induced skeletal muscle atrophy. Am J Physiol Endocrinol Metab. 2014;307:E983-E993. doi: 10.1152/ajpendo.00234.2014. [DOI] [PubMed] [Google Scholar]
- 74. Agarwal S, Mirzoeva S, Readhead B, Dudley JT, Budunova I. PI3K inhibitors protect against glucocorticoid-induced skin atrophy. EBioMedicine. 2019;41:526-537. doi: 10.1016/j.ebiom.2019.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lan YC, Chang CL, Sung MT, et al. Zoledronic acid-induced cytotoxicity through endoplasmic reticulum stress triggered REDD1-mTOR pathway in breast cancer cells. Anticancer Res. 2013;33:3807-3814. [PubMed] [Google Scholar]
- 76. Yun S-M, Woo SH, Oh ST, et al. Melatonin enhances arsenic trioxide-induced cell death via sustained upregulation of Redd1 expression in breast cancer cells. Mol Cell Endocrinol. 2016;422:64-73. doi: 10.1016/j.mce.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 77. Tirado-Hurtado I, Fajardo W, Pinto JA. DNA damage inducible transcript 4 gene: the switch of the metabolism as potential target in cancer. Front Oncol. 2018;8:106. doi: 10.3389/fonc.2018.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pinto JA, Rolfo C, Raez LE, et al. In silico evaluation of DNA damage inducible transcript 4 gene (DDIT4) as prognostic biomarker in several malignancies. Sci Rep. 2017;7:1526. doi: 10.1038/s41598-017-01207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]