Abstract
Oral microbiomes vary in cariogenic potential; these differences may be established early in life. A major concern is whether mothers transmit cariogenic bacteria to their children. Here we characterize early salivary microbiome development and the potential associations of that development with route of delivery, breastfeeding, and mother’s oral health, and we evaluate transmission of microbes between mother and child. We analyzed saliva and metadata from the Center for Oral Health Research in Appalachia. For this cohort study, we sequenced the V6 region of the 16S rRNA gene and used quantitative polymerase chain reaction to detect Streptococcus mitis, Streptococcus sobrinus, Streptococcus mutans, Streptococcus oralis, and Candida albicans in the saliva from mothers and their infants, collected at 2, 9, and 12 mo (Pennsylvania site) and 2, 12, and 24 mo (West Virginia site). Breastfed children had lower relative abundances of Prevotella and Veillonella. If mothers had decayed, missing, or filled teeth, children had greater abundances of Veillonella and Actinomyces. There was little evidence of maternal transmission of selected microbes. At 12 mo, children’s microbiomes were more similar to other children’s than to their mothers’. Infants’ salivary microbiomes became more adult-like with age but still differed with mothers’ microbiomes at 12 mo. There was little evidence supporting transmission of selected microbes from mothers to children, but risk of colonization was associated with tooth emergence. Children are likely to acquire cariogenic bacteria from a variety of sources, including foods and contact with other children and adults.
Keywords: epidemiology, caries, gingivitis, 16S rRNA, early childhood caries, oral microbiome
Background
In 2015, early childhood caries (ECC) affected 21.4% of US children (Fleming and Afful 2018). Children with ECC are more likely to develop subsequent caries in their primary and permanent teeth (Berkowitz 2003; Leong et al. 2013). Teng et al. (2015) used temporal patterns of the salivary microbiome to predict onset of ECC among children followed from age 4 to 6 y, but studies describing salivary microbiome dynamics from infancy to age 2 y are few. Two Swedish cohorts revealed that the oral microbiome increases in alpha diversity during early childhood and that microbiome composition is associated with breastfeeding and route of delivery (Dzidic, Abrahamsson, et al. 2018; Dzidic, Collado, et al. 2018; Kennedy et al. 2019). To date, there are no similar studies conducted in US high-risk populations.
Also of interest is whether mothers transmit cariogenic bacteria to their children, putting them at higher risk of dental caries (previously reviewed by De Abreu Da Silva Bastos et al. 2015). We found no studies that assessed transmission of the oral microbiome and specific microbes from mother to child. Several microbes are of interest. Streptococcus mutans and Streptococcus sobrinus have a known association with dental caries (Kishi et al. 2009), and Candida albicans is detected more frequently in children with ECC than in caries-free children (Xiao, Huang, et al. 2018). Furthermore, C. albicans often occurs with S. mutans in plaque biofilms from children with dental caries (de Carvalho et al. 2006). Streptococcus mitis and Streptococcus oralis are commensals but may enhance colonization of tooth surfaces by other microbes; they also have a potentially mutualistic relationship with C. albicans (Bertolini and Dongari-Bagtzoglou 2019).
Our study closes these gaps by characterizing the development of the salivary microbiome in 101 healthy infants from a cohort in Appalachia at high risk of caries (Neiswanger et al. 2015). We also compare the salivary microbiomes of mothers and infants and the presence of selected microbes over time, as assessed with quantitative polymerase chain reaction (qPCR). Three main hypotheses are evaluated: 1) the infant salivary microbiome is dynamic but stabilizes over time; 2) the route of delivery and the duration of breastfeeding are associated with bacterial diversity and composition; and 3) selected microbes are transmitted from mother to child. While our results confirm the first 2 hypotheses and are consistent with the literature, we did not find strong evidence supporting transmission of microbiome composition or specific species from mother to child. Acquisition of selected microbes was more associated with age-dependent development.
Methods
Study Population and Protocol
We studied a subset of 101 mother-infant pairs from the 1,172 participants in the COHRA 2 cohort (Center for Oral Health Research in Appalachia; Neiswanger et al. 2015). COHRA 2 recruited pregnant Caucasian women aged >17 y between 12- and 29-wk gestation, excluding those who had tuberculosis or were immunocompromised. Only infants born at ≥35-wk gestation without any serious medical conditions were included.
A trained, calibrated dental professional assessed each participant’s oral health status and collected oral specimens. Formal training/calibration sessions were conducted on a regular basis for examiners from all sites. Interrater reliability scores (Cohen’s kappa) for sound, decayed, and filled group surface codes ranged from 82.1 to 92.5. Specific protocols for the clinical examination and calibration are described elsewhere (Neiswanger et al. 2015).
Mothers were instructed not to eat or brush their teeth for 1 h before their visit and not to feed their infants 1 h before the infant dental examination. Saliva was collected from mothers during their dental examination, usually ≥2 h into their appointment. Saliva was collected with OMNIgene Discover OM-501 or OM-505 collection kits (DNA Genotek Inc.) either by spitting (mothers) or swabbing DNA Genotek sponge swabs (infants). COHRA research staff and the University of Pittsburgh Center for Social and Urban Research conducted in-person and telephone surveys regarding mothers’ oral health, demographics, and breastfeeding.
Participating mothers gave informed consent on behalf of themselves and their infants. The study protocol was reviewed and approved by the institutional review boards of the University of Pittsburgh and West Virginia University. This study conforms to STROBE guidelines (Strengthening the Reporting of Observational Studies in Epidemiology) for cohort studies.
Sample Selection
We selected a sample of 101 mothers and their infants who completed the 2-mo, first-tooth (mean age, 9 mo), and 12-mo postpartum visits in Pennsylvania (n = 77) and the 2-mo, 12-mo, and 24-mo postpartum visits in West Virginia (n = E24). All 2-mo visits occurred between 2012 and 2013. All eligible women without dental caries at enrollment were included (n = 21), and the remaining 80 women were randomly sampled from a list ordered by number of decayed, missing, or filled teeth (DMFT) to represent caries distribution at enrollment. For this study, the presence of white spots was not considered carious. Samples were selected for each infant at all 3 time points, while samples were selected for each mother at 2 time points (Fig. 1). In total, 505 samples were included.
Figure 1.
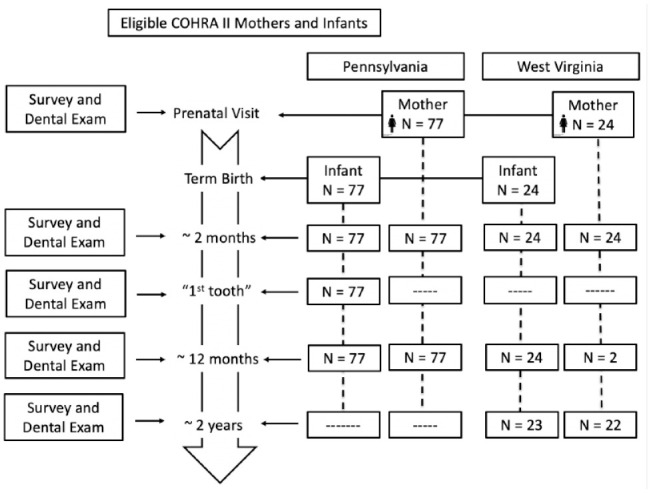
Flowchart of study participants and selected samples during the postpartum period. Mothers and infants participating in the COHRA 2 (Center for Oral Health Research in Appalachia) from Pennsylvania (n = 154) and West Virginia (n = 48).
Sequence Generation and Processing
DNA was extracted from saliva samples, and the V6 region of the 16s rRNA gene was amplified with a modified version of primers and protocols described elsewhere (Gloor et al. 2010; Foxman et al. 2016). Two technical replicates were sequenced on separate lanes of an Illumina HiSeq platform with 100 paired-end cycles at the University of Michigan Sequencing Core.
Paired ends were joined with FLASH; sequences were quality filtered and de-multiplexed with the split_libraries.py script in QIIME 1.9.0. This resulted in 98,582,469 sequences with a mean length of 124 base pairs. The reads were clustered into oligotypes with an unsupervised minimum entropy decomposition method (Eren et al. 2015). The minimum substantive abundance of an oligotype (–M) criterion was set to 19,706, and the maximum variation allowed in each node (–V) criterion was set to 1. Of the total 98,582,469 reads, minimum entropy decomposition removed 21,724,727 reads due to the –M and –V criteria and clustered the remaining 76,803,742 reads into 366 oligotypes. Taxonomy for each oligotype was assigned to the CORE reference database via the RDP consensus taxonomy assigner in QIIME 1.9.0. CORE is a specialized database for the identification of bacteria within the oral microbiome (Griffen et al. 2011). Raw sequences were deposited in the NCBI Sequence Read Archive (PRJNA612517).
Community State Type Generation
Community state types (CSTs) were identified with an unsupervised clustering method (DirichletMultinomial package in R version 3.3.0; R Foundation for Statistical Computing), and the number of CSTs was determined by selecting the least number of Dirichlet components that provided the lowest Laplace approximation of model fit (Holmes et al. 2012). To enhance to robustness of the developed CST, we combined sequence data from the 101 mothers and infants with sequence data obtained with the same library preparation and sequencing protocols from a previous published study of the COHRA 1 cohort (Foxman et al. 2016).
Each sample was assigned to 1 community state based on maximum posterior probability ≥80% for the most probable community state. All samples in this data set were classified with this criterion.
Quantitative Polymerase Chain Reaction
We screened saliva DNA samples for S. mitis, S. sobrinus, S. mutans, S. oralis, and C. albicans with conserved qPCR protocols and primers described elsewhere (Yoshida et al. 2003; Rosenthal et al. 2013; Henne et al. 2014; Appendix Table 1). The total reaction volume was 10 µL, consisting of 5:2.5:2:0.5 parts SYBR Green PCR Master Mix (Sso EvaGreen Supermix; Bio-Rad), DNA template, nuclease-free water, and forward and reverse primer pair at 5µM concentration, respectively. The limit of detection was 100 genomic copies per milliliter; samples below this threshold were considered negative. Assays were run in duplicate, with a positive and negative control in each run. Samples with discordant duplicates were retested.
We imputed values for the 122 samples with no remaining saliva available for qPCR testing (Enders 2010). As a sensitivity analysis, we compared the results from the analysis with imputed data to another analysis excluding samples with missing data (Appendix Fig. 1). The interpretation of the 2 analyses were the same, so we present the imputed results.
Statistical Analysis
We used the Phyloseq 1.10.0 R package and QIIME 1.9.0 commands to calculate Shannon’s diversity index (alpha diversity) and Bray-Curtis distances (beta diversity). Differences in Shannon diversity and Bray-Curtis distance by group were assessed with the Wilcoxon rank sum nonparametric test; a P value <0.05 was considered significant. We calculated transition rates among CSTs for those with all consecutive visits, separately by site (West Virginia, n = 22; Pennsylvania, n = 77).
To understand factors associated with transitioning among community states, we fit a series of linear mixed effects models. To account for repeated measures and the nested hierarchical structure, random effects were included for each infant with the lme4 package in R. Separate models were fit to assess the associations of primary exposures with the log-transformed relative abundance of the top 10 genera adjusted for confounders: age (continuous) and collection site (Pennsylvania vs. West Virginia). Bootstrap confidence intervals were calculated with 1,000 simulations per model.
Mean log abundance of the 4 selected Streptococcus species and Candida albicans was calculated for each CST in each age group. To estimate mother-infant transmission, we calculated the proportion of infant samples that were positive at the 2- and 12-mo visits given that their mothers ever tested positive.
We compared the microbial communities found among mothers and infants using ALDEx2 (Fernandes et al. 2013). We used the Benjamini-Hochberg procedure (Benjamini and Hochberg 1995) to correct for multiple comparisons and set the false discovery–corrected P value significance to <0.05. All statistical analyses were conducted in R version 3.3.0.
Results
We included 77 mother-infant pairs from Pennsylvania and 24 from West Virginia, followed at 3 time points. The schedule for both sites included 2- and 12-mo visits (Fig. 1): samples from Pennsylvania infants also visited at the time of first tooth emergence (~9 mo). The West Virginia protocol did not include a 9-mo visit, so we included samples from the 24-mo visit. Most infants were delivered vaginally and fed breast and formula milk, and their mothers had a DMFT score ≥1. Mothers in the 2 sites were similar in education and income (Table).
Table.
Baseline Characteristics of 101 COHRA 2 Infants and Their Mothers from the 2 Study Sites: Pennsylvania and West Virginia.
| Participants, n (%) | ||
|---|---|---|
| Characteristic | Pennsylvania (n = 77) | West Virginia (n = 24) |
| Infants | ||
| Delivery route | ||
| Vaginal | 53 (68.8) | 16 (66.7) |
| C-Section | 22 (28.6) | 8 (33.3) |
| Missing | 2 (2.6) | 0 (0) |
| Milk fed at 2 mo | ||
| Breast milk | 18 (23.4) | 3 (12.5) |
| Formula milk | 24 (31.2) | 13 (54.2) |
| Breast and formula milk | 35 (45.5) | 7 (29.2) |
| Missing | 0 (0) | 1 (4.2) |
| Mothers | ||
| Oral health status: baseline DMFT | ||
| 0 | 19 (24.7) | 2 (8.3) |
| 1 to 7 | 31 (40.2) | 12 (50.0) |
| ≥8 | 27 (35.1) | 10 (41.7) |
| Education status | ||
| Less than high school diploma | 4 (5.2) | 4 (16.7) |
| High school graduate or GED | 9 (11.7) | 5 (20.8) |
| Some college, no degree | 16 (20.8) | 5 (20.8) |
| Associate or bachelor degree | 32 (41.6) | 7 (29.2) |
| Master, PhD, professional degree | 16 (20.8) | 2 (8.3) |
| Missing | 0 (0) | 1 (4.2) |
| Household income, $ | ||
| <25,000 | 18 (24) | 8 (33.3) |
| 25,000 to 49,999 | 13 (17.3) | 8 (33.3) |
| 50,000 to 74,999 | 13 (17.3) | 4 (16.7) |
| 75,000 to 99,999 | 15 (20) | 2 (8.3) |
| ≥100,000 | 16 (21.3) | 1 (4.2) |
| Missing | 0 (0) | 1 (4.2) |
Pairs were based on mothers’ oral health status. Those with no decayed, missing, or filled teeth (DMFT) were oversampled. The remaining were selected via a systematic sample from a list ordered by DMFT. There was no statistically significant difference (at P < 0.05 level) between study sites at baseline. Some percentages add to 100.1% due to rounding.
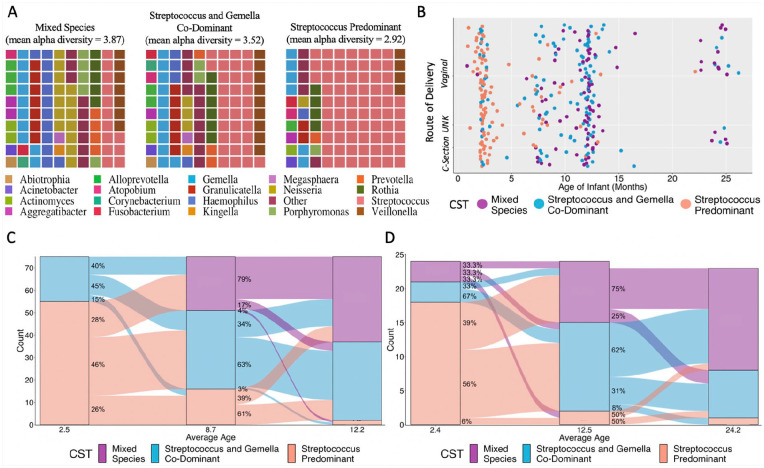
Salivary microbiomes from mothers and infants were classified into 1 of 4 CSTs. No infants had the most diverse and least variable adult CST, so it was removed from downstream analysis (Appendix Fig. 2). The included 3 CSTs were labeled as follows: mixed species, Streptococcus and Gemella codominant, and Streptococcus predominant. CSTs varied in alpha diversity (Fig. 2A).
Figure 2.
Characteristics of observed community state types (CSTs) and transition probabilities of changing among CSTs during follow-up. (A) Waffle plots of the 3 CSTs observed among infants; observed mean alpha diversity (Shannon index) is noted. (B) Changes in salivary CSTs by route of delivery with infant age: vaginal (n = 69), cesarean section (n = 30), unknown (n = 2). (C) Dynamics of salivary CSTs among 2-, 6-, and 12-mo visits of Pennsylvania infants (n = 77). (D) Dynamics of CSTs among 2-, 12-, and 24-mo visits of West Virginia infants (n = 24, n = 22 at 24 mo). Infants participating in the COHRA 2 (Center for Oral Health Research in Appalachia) from Pennsylvania and West Virginia.
Changes in the Salivary Microbiome with Age
Microbiome Shannon’s diversity and richness (Chao1) increased with infant age. This was consistent across groups defined by mothers’ oral health status, education, delivery route, and whether the infant was breastfed (Fig. 2B, Appendix Figs. 3 and 4). A similar pattern was observed with CSTs (Fig. 2C, D). Once in the mixed or Streptococcus and Gemella codominant CSTs, 97.3% of Pennsylvania infants and 95.7% of West Virginia infants remained there.
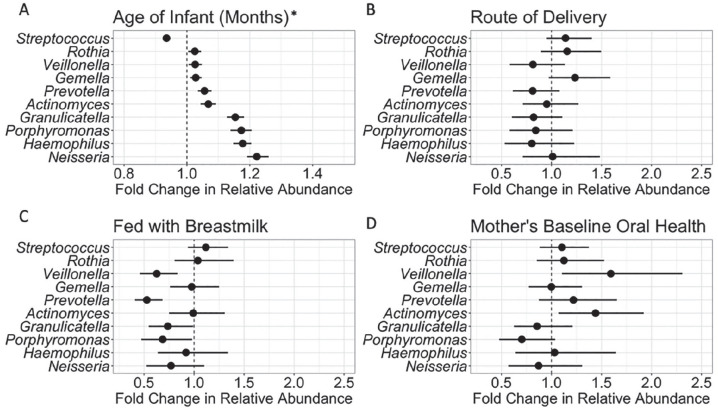
We explored whether the individual genera that most determined CSTs also varied by age, by fitting a series of linear mixed models predicting the log-transformed relative abundance of each genera adjusted for geographic area (Fig. 3A). The relative abundance of Streptococcus decreased by 6.5% with each additional month of age (P < 0.00001); by contrast, the relative abundance of Granulicatella, Porphyromonas, Haemophilus, and Neisseria increased by 15.4%, 17.3%, 17.7%, and 22.2%, respectively, with each month of age (P < 0.00001). A 6-mo increase in age corresponded to a 123% increase in the relative abundance of Neisseria.
Figure 3.
Models of log-transformed relative abundance of the top 10 genera (continuous) adjusted for collection site (Pennsylvania vs. West Virginia), repeated measures over time, and selected variables. (A) Infant age (months). Note that the confidence limits for Streptococcus are too small to be visualized on the figure (odds ratio, 0.94; 95% CI, 0.93 to 0.95). (B) Route of delivery (categorical) adjusted for infant age. (C) Breastfeeding status (binary) adjusted for infant age and route of delivery. (D) Mother’s oral health status (categorical) at baseline adjusted for infant age. Infants participating in the COHRA 2 (Center for Oral Health Research in Appalachia) from Pennsylvania and West Virginia (303 observations from 101 individuals). *Panel A x-axis scale differs from panels B, C, and D.
Associations of Salivary Microbiome with Route of Delivery, Breastfeeding, and Mother’s Oral Health
At 2 mo, the salivary microbiomes of infants delivered by cesarean section were significantly more diverse than those delivered vaginally (Shannon diversity index: 3.05 vs. 2.92, P = 0.027), but these differences disappeared by 12 mo (P = 0.26), though not in CST distribution at either period (2 mo, P = 0.958; 12 mo, P = 0.524). After adjusting for age and geographic area, infants delivered via cesarean section as compared with vaginally had increased relative abundances of Streptococcus, Rothia, and Gemella of 13.9%, 15.6%, and 23.4%, respectively, and decreased abundances of Granulicatella, Porphyromonas, and Haemophilus of 17.9%, 15.8%, and 19.7% (Fig. 3B).
The salivary microbiome of babies fed only breast milk at 2 mo had lower diversity than babies fed only formula (Shannon diversity: 2.77 vs. 3.12, P = 0.003) and those fed breast milk only versus breast milk and formula fed (2.77 vs. 2.92; P = 0.293). These differences did not persist over time: by 12 mo, the Shannon diversity by breastfeeding type was similar (breast milk only, 3.69; breast milk and formula, 3.78; formula only, 3.75). After adjusting for age, delivery route, and geographic area, infants who were breastfed as compared with those not breastfed had decreased relative abundances of Veillonella (37.3%), Prevotella (47.3%), Granulicatella (26.4%), and Porphyromonas (31.4%; Fig. 3C).
Although we observed no significant changes in the alpha diversity of infants’ salivary microbiome by mothers’ baseline oral health (Shannon diversity, 3.33 vs. 3.41 for DMFT >0 vs. 0; P = 0.31), after adjusting for age, there were differences in the relative abundance of the most common taxa (Appendix Figs. 2–4). Among infants whose mothers had a DMFT >0 versus 0, the relative abundance of Veillonella and Actinomyces increased by 59.2% and 44.0%, respectively (Fig. 3D).
Transmission from Mother to Infant
We examined transmission from mother to infant with the microbiome and qPCR for specific organisms. The mean Bray-Curtis distance—where 0 corresponds to having the same microbes present and 1 to sharing no microbes between children and mothers—decreased with age (Pennsylvania: 2 mo, 0.68; 12 mo, 0.60; P = 0.0004; West Virginia: 2 mo, 0.72; 24 mo, 0.55; P = 0.0012). However, the mean Bray-Curtis distance among children at 12 and 24 mo was smaller than the distance between mothers and children (Pennsylvania, 12 mo: among children, 0.53; mothers-children, 0.60; P = 0.00002; West Virginia, 24 mo: among children, 0.51; mothers-children, 0.55; P = 0.173; Appendix Fig. 5).
To assess whether the relative abundance of any taxa significantly differed between children aged 12 mo and their mothers at 12 mo postpartum, we used ALDEx2, identifying taxa with a Benjamini-Hochberg-corrected P value <0.05 and an effect size >1. The following taxa were more abundant in children: Prevotella, Megasphaera, Atopobium, Campylobacter, TM7, Selenomonas, and Solobacterium. In contrast, Gemella, Granulicatella, Acinetobacter, and Enterococcus were more abundant among their mothers (Appendix Table 2).
We also estimated the probability of transmission of C. albicans, S. mutans, S. mitis, S. oralis, and S. sobrinus from mother to infant (Fig. 4). We considered the mother to be colonized with a taxa if there 100 copy numbers were detected by qPCR at any visit. Mothers in West Virginia had higher prevalence of all taxa (Appendix Table 3). There were no clear associations, however, with the presence of selected taxa in the mothers and the colonization of their children in either site.
Figure 4.
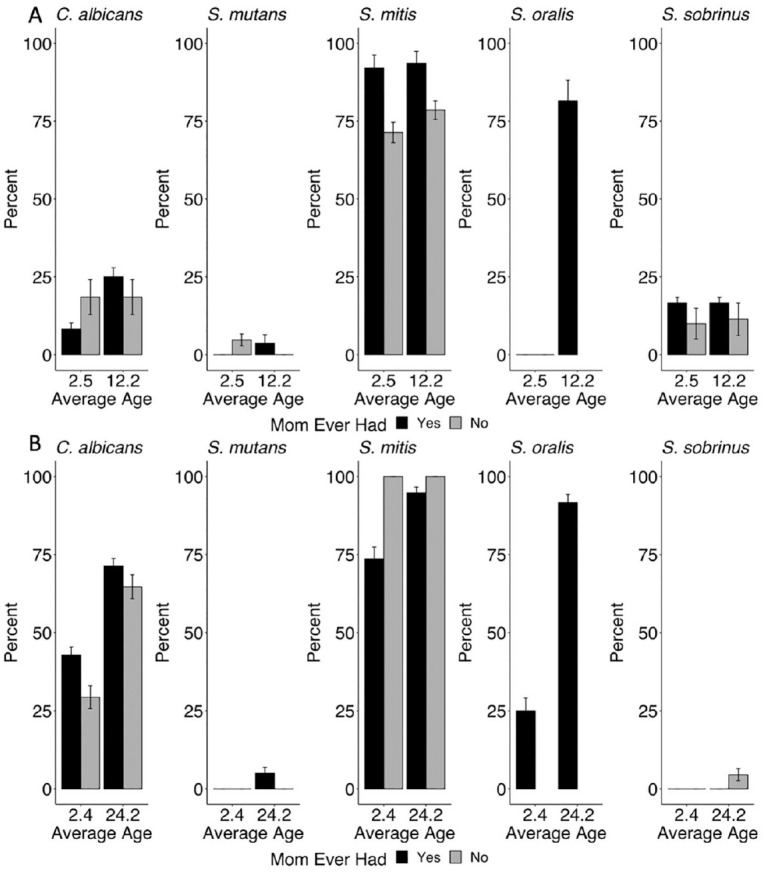
Probability of transmission from mother to infant of selected species by infant age (months), given that mother tested positive at any time point: (A) Pennsylvania (n = 77) and (B) West Virginia (n = 24). Values were imputed for 122 of 505 samples. Mothers and infants participating in the COHRA 2 (Center for Oral Health Research in Appalachia) from Pennsylvania and West Virginia. Error bars indicate 95% CIs.
Discussion
In this prospective cohort of 101 infants measured at 3 time points, we had 3 primary findings. First, the salivary microbiome of infants increased in alpha diversity as the infants aged, and age was associated with the prevalence and abundance of selected microbes within the oral cavity. Second, after adjusting for infant age, there were differences in community state and specific taxa among infants who were breastfed versus formula fed and infants whose mothers had caries versus mothers who did not. Third, although infants’ salivary microbiomes became more adult-like with age, there were clear differences in the infants’ microbiomes versus those of their mothers, and there was little evidence of transmission of selected microbes between mothers and their infants across time points.
Although using the V6 region of the 16S rRNA gene provides somewhat less resolution to the species level than other variable regions, our observed changes in salivary microbiome diversity with age are consistent with results of a Swedish study of 90 children sampled at 3, 6, 12, and 24 mo (Dzidic, Collado, et al. 2018) and a Swedish study of 59 children sampled at 6, 12, and 24 mo of age (Kennedy et al. 2019). Like these studies, we found that the relative abundance of Streptococcus spp. decreases with age and that Neisseria increases with age as part of a normal ecologic succession, and we observed microbiome differences by route of delivery and breastfeeding.
Transfer of microbes from mother to newborn occurs during labor and delivery; the oral microbiota in vaginally delivered infants differs from those delivered via cesarean (Dunn et al. 2017; Li et al. 2018). Breast milk selects for specific microbes (Milani et al. 2017), and several studies have demonstrated that children who are breastfed are at lower risk of ECC than children who are bottle fed (reviewed by Avila et al. 2015). However, once breastfeeding ceases, there are other ecologic niches available to be filled by microbes in the environment.
Our study expands our understanding of salivary microbiome development, as we characterized how breastfeeding, route of delivery, and presence of dental caries in the mother were associated with taxa relative abundance independent of age. Like the Swedish study of 90 children (Dzidic, Collado, et al. 2018), we found a lower relative abundance of caries-associated bacterial species—Veillonella, Prevotella, and Porphyromonas—among breastfed infants. In our study, the relative abundance of Veillonella and Actinomyces increased among infants whose mothers had dental caries. We found no previous studies for comparison. As these species are found more frequently in the saliva of children with caries (Jiang et al. 2016), replication is warranted.
Although dental caries are not transmissible (Twetman 2018), there has been considerable concern that transmission of cariogenic bacteria from mothers to offspring may increase risk of early onset of caries (Damle et al. 2016). Furthermore, early colonization of S. mutans is related to high caries incidence in childhood (Law et al. 2007). Multiple reports suggest transmission of S. mutans between mothers and their infants (reviewed by De Abreu Da Silva Bastos et al. 2015), but the majority are cross-sectional. A weakness of most of these studies—shared by ours—is that S. mutans were classified by species only, so strain differences were not detected. However, the longitudinal nature of our study is a strength.
Our study is the first report of changes in C. albicans colonization with age and the first to assess the association with the mother’s colonization. C. albicans is associated with a highly acidogenic and acid-tolerant bacterial community in children with severe ECC (Xiao, Grier, et al. 2018; Fakhruddin et al. 2020). Our results suggest that colonization with S. mutans and C. albicans is primarily a result of age-related changes in exposure (e.g., introduction of foods, interactions with other children) and potential for colonization (i.e., emergence of teeth) rather than transmission from mother to child.
Conclusion
The salivary microbiome is dynamic during the first 2 y of life, is influenced by whether the child was breastfed, and is associated with maternal oral health status. Differences by delivery mode were no longer apparent after 12 mo. Multiple environmental factors are responsible for the assembly of the salivary microbiome in infancy; age-related factors were the strongest determinants.
Author Contributions
K. Ramadugu, C.F. Marrs, B. Foxman, contributed to conception, design, and data analysis, drafted and critically revised the manuscript; D. Bhaumik, T. Luo, R.E. Gicquelais, K.H. Lee, E.B. Stafford, contributed to data analysis, critically revised the manuscript; K. Neiswanger, D.W. McNeil, M.L. Marazita, contributed to conception and design, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034520947665 for Maternal Oral Health Influences Infant Salivary Microbiome by K. Ramadugu, D. Bhaumik, T. Luo, R.E. Gicquelais, K.H. Lee, E.B. Stafford, C.F. Marrs, K. Neiswanger, D.W. McNeil, M.L. Marazita and B. Foxman in Journal of Dental Research
Acknowledgments
We thank the women and children who participated in the COHRA 2 project. We also express our sincere thanks to field staff past and present in West Virginia and Pennsylvania for their dedicated efforts. We thank Elizabeth Stafford for laboratory assistance and our colleagues in the Center for Molecular and Clinical Epidemiology at the University of Michigan School of Public Health and in the COHRA for their comments and guidance on this work. An earlier version of parts of this study was presented at the 13th International Conference on Molecular Epidemiology and Evolutionary Genetics of Infectious Diseases and at the 16th International Symposium on Microbial Ecology.
Footnotes
A supplemental appendix to this article is available online.
This work was funded by the National Institutes of Health (R01-DE014899).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iDs: R.E. Gicquelais  https://orcid.org/0000-0002-7022-6385
https://orcid.org/0000-0002-7022-6385
K.H. Lee  https://orcid.org/0000-0002-0657-1483
https://orcid.org/0000-0002-0657-1483
D.W. McNeil  https://orcid.org/0000-0002-0766-8455
https://orcid.org/0000-0002-0766-8455
B. Foxman  https://orcid.org/0000-0001-6682-238X
https://orcid.org/0000-0001-6682-238X
Data Deposition: Raw sequencing data are being deposited in an NCBI Sequence Read Archive (PRJNA612517).
References
- Avila WM, Pordeus IA, Paiva SM, Martins CC. 2015. Breast and bottle feeding as risk factors for dental caries: a systematic review and meta-analysis. PLoS One. 10(11):e0142922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 57(1):289–300. [Google Scholar]
- Berkowitz RJ. 2003. Causes, treatment and prevention of early childhood caries: a microbiologic perspective. J Can Dent Assoc. 69(5):304–307. [PubMed] [Google Scholar]
- Bertolini M, Dongari-Bagtzoglou A. 2019. The relationship of Candida albicans with the oral bacterial microbiome in health and disease. Adv Exp Med Biol. 1197:69–78. [DOI] [PubMed] [Google Scholar]
- De Abreu Da Silva Bastos V, Freitas-Fernandes LB, Da Silva Fidalgo TK, Martins C, Mattos CT, De Souza IPR, Maia LC. 2015. Mother-to-child transmission of Streptococcus mutans: a systematic review and meta-analysis. J Dent. 43(2):181–191. [DOI] [PubMed] [Google Scholar]
- de Carvalho FG, Silva DS, Hebling J, Spolidorio LC, Spolidorio DMP. 2006. Presence of mutans streptococci and Candida spp. in dental plaque/dentine of carious teeth and early childhood caries. Arch Oral Biol. 51(11):1024–1028. [DOI] [PubMed] [Google Scholar]
- Damle SG, Yadav R, Garg S, Dhindsa A, Beniwal V, Loomba A, Chatterjee S. 2016. Transmission of mutans streptococci in mother-child pairs. Indian J Med Res. 144(2):264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AB, Jordan S, Baker BJ, Carlson NS. 2017. The maternal infant microbiome: considerations for labor and birth. MCN Am J Matern Nurs. 42(6):318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzidic M, Abrahamsson TR, Artacho A, Collado MC, Mira A, Jenmalm MC. 2018. Oral microbiota maturation during the first 7 years of life in relation to allergy development. Allergy. 73(10):2000–2011. [DOI] [PubMed] [Google Scholar]
- Dzidic M, Collado MC, Abrahamsson T, Artacho A, Stensson M, Jenmalm MC, Mira A. 2018. Oral microbiome development during childhood: an ecological succession influenced by postnatal factors and associated with tooth decay. ISME J. 12(9):2292–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK. 2010. Applied missing data analysis. New York (NY): Guilford Press. [Google Scholar]
- Eren AM, Morrison HG, Lescault PJ, Reveillaud J, Vineis JH, Sogin ML. 2015. Minimum entropy decomposition: unsupervised oligotyping for sensitive partitioning of high-throughput marker gene sequences. ISME J. 9(4):968–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhruddin KS, Perera Samaranayake L, Egusa H, Chi Ngo H, Panduwawala C, Venkatachalam T, Kumarappan A, Pesee S. 2020. Candida biome of severe early childhood caries (S-ECC) and its cariogenic virulence traits.J Oral Microbiol. 12(1):1724484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes AD, Macklaim JM, Linn TG, Reid G, Gloor GB. 2013. ANOVA-like differential expression (ALDEx) analysis for mixed population RNA-Seq. PLoS One. 8(7):e67019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming E, Afful J. 2018. Prevalence of total and untreated dental caries among youth: United States, 2015–2016. NCHS Data Brief. 2018;(307):1–8. [PubMed] [Google Scholar]
- Foxman B, Luo T, Srinivasan U, Ramadugu K, Wen A, Goldberg D, Shedden K, Crout R, McNeil DW, Weyant R, et al. 2016. The effects of family, dentition, and dental caries on the salivary microbiome. Ann Epidemiol. 26(5):348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor GB, Hummelen R, Macklaim JM, Dickson RJ, Fernandes AD, MacPhee R, Reid G. 2010. Microbiome profiling by illumina sequencing of combinatorial sequence-tagged PCR products. PLoS One. 5(10):e15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffen AL, Beall CJ, Firestone ND, Gross EL, Difranco JM, Hardman JH, Vriesendorp B, Faust RA, Janies DA, Leys EJ. 2011. CORE: a phylogenetically-curated 16S rDNA database of the core oral microbiome. PLoS One. 6(4):e19051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne K, Li J, Stoneking M, Kessler O, Schilling H, Sonanini A, Conrads G, Horz HP. 2014. Global analysis of saliva as a source of bacterial genes for insights into human population structure and migration studies. BMC Evol Biol. 14(1):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes I, Harris K, Quince C. 2012. Dirichlet multinomial mixtures: generative models for microbial metagenomics. PLoS One. 7(2):e30126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Gao X, Jin L, Lo ECM. 2016. Salivary microbiome diversity in caries-free and caries-affected children. Int J Mol Sci. 17(12):1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy B, Peura S, Hammar U, Vicenzi S, Hedman A, Almqvist C, Andolf E, Pershagen G, Dicksved J, Bertilsson S, et al. 2019. Oral microbiota development in early childhood. Sci Rep. 9(1):19025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi M, Abe A, Kishi K, Ohara-Nemoto Y, Kimura S, Yonemitsu M. 2009. Relationship of quantitative salivary levels of Streptococcus mutans andS. sobrinus in mothers to caries status and colonization of mutans streptococci in plaque in their 2.5-year-old children. Community Dent Oral Epidemiol. 37(3):241–249. [DOI] [PubMed] [Google Scholar]
- Law V, Seow WK, Townsend G. 2007. Factors influencing oral colonization of mutans streptococci in young children. Aust Dent J. 52(2):93–100. [DOI] [PubMed] [Google Scholar]
- Leong PM, Gussy MG, Barrow SYL, De Silva-Sanigorski A, Waters E. 2013. A systematic review of risk factors during first year of life for early childhood caries. Int J Paediatr Dent. 23(4):235–250. [DOI] [PubMed] [Google Scholar]
- Li H, Wang J, Wu L, Luo J, Liang X, Xiao B, Zhu Y. 2018. The impacts of delivery mode on infant’s oral microflora. Sci Rep. 8(1):11938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, Belzer C, Delgado Palacio S, Arboleya Montes S, Mancabelli L, et al. 2017. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. 81(4):e00036-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiswanger K, McNeil DW, Foxman B, Govil M, Cooper ME, Weyant RJ, Shaffer JR, Crout RJ, Simhan HN, Beach SR, et al. 2015. Oral health in a sample of pregnant women from Northern Appalachia (2011–2015). Int J Dent. 2015:469376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal M, Aiello A, Larson E, Chenoweth C, Foxman B. 2013. Healthcare workers’ hand microbiome may mediate carriage of hospital pathogens. Pathogens. 3(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng F, Yang F, Huang S, Bo C, Xu ZZ, Amir A, Knight R, Ling J, Xu J. 2015. Prediction of early childhood caries via spatial-temporal variations of oral microbiota. Cell Host Microbe. 18(3):296–306. [DOI] [PubMed] [Google Scholar]
- Twetman S. 2018. Prevention of dental caries as a non-communicable disease. Eur J Oral Sci. 126 Suppl 1:19–25. [DOI] [PubMed] [Google Scholar]
- Xiao J, Grier A, Faustoferri RC, Alzoubi S, Gill AL, Feng C, Liu Y, Quivey RG, Kopycka-Kedzierawski DT, Koo H, et al. 2018. Association between oral candida and bacteriome in children with severe ECC. J Dent Res. 97(13):1468–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Huang X, Alkhers N, Alzamil H, Alzoubi S, Wu TT, Castillo DA, Campbell F, Davis J, Herzog K, et al. 2018. Candida albicans and early childhood caries: a systematic review and meta-analysis. Caries Res.52(1–2):102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Suzuki N, Nakano Y, Kawada M, Oho T, Koga T. 2003. Development of a 5′ nuclease-based real-time PCR assay for quantitative detection of cariogenic dental pathogens Streptococcus mutans and Streptococcus sobrinus. J Clin Microbiol. 41(9):4438–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034520947665 for Maternal Oral Health Influences Infant Salivary Microbiome by K. Ramadugu, D. Bhaumik, T. Luo, R.E. Gicquelais, K.H. Lee, E.B. Stafford, C.F. Marrs, K. Neiswanger, D.W. McNeil, M.L. Marazita and B. Foxman in Journal of Dental Research