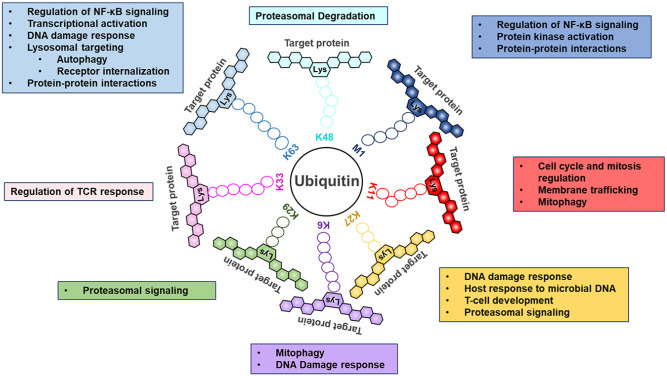
Figure 2.
Individual ubiquitin linkage types and their associated biological roles. Ubiquitin, a small 76–amino acid protein, can be attached to a targeted substrate or a ubiquitin molecule that is already attached to a substrate, resulting in specific polyubiquitin linkage types. In a ubiquitin chain, ubiquitin moieties can be conjugated through one of their lysine resides (Lys11, Lys27, Lys6, Lys29, Lys33, Lys63, and Lys48) or the N-terminal methionine residue (Met1). Each chain is recognized by different ubiquitin-binding domains, targeting proteins in specific signaling pathways. Most extensively studied, Lys48- and Lys63-linked chains are the 2 most abundant chain types and regulate proteasomal degradation and a variety of proteolytic and nonproteolytic events, respectively. Recently, innovative technologies revealed the role of the remaining ubiquitin chain types in controlling cellular processes ranging from cell cycle control to cytokine signaling (Swatek and Komander 2016; Mendes et al. 2020).