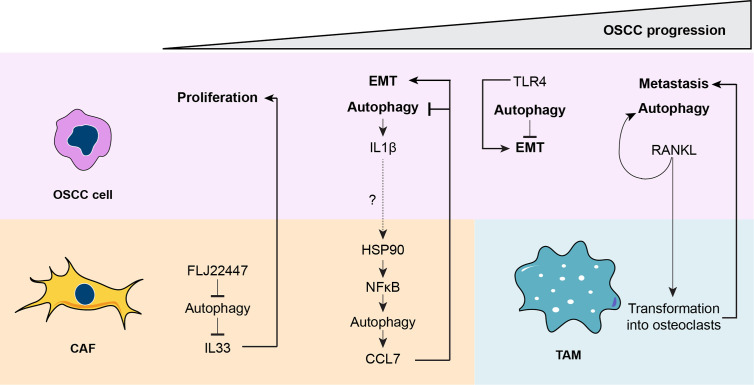
Figure 4.
A unified model for the role of autophagy during oral squamous cell carcinoma (OSCC) progression. During the early stages of OSCC, the long non-coding RNA FLJ22447 inhibits autophagy in cancer-associated fibroblasts (CAFs), impairing the autophagic degradation of IL33. Increased levels of IL33 are released from CAFs, inducing proliferation of the OSCC cells. Then, OSCC cells increase autophagy that releases interleukin-1β (IL1β), which then increases autophagy in CAFs through an nuclear factor κB ((NFκB)-dependent mechanism. The IL1β released from the OSCC cells may promote activation of heat shock protein 90 (HSP90) in the CAFs to increase NFκB activity, but this needs to be demonstrated (dashed line). However, it is known that NFκB-dependent autophagy in CAFs induces release of chemokine (C-C motif) ligand 7 (CCL7), which acts on OSCC cells, promoting epithelial-mesenchymal transition (EMT) and inhibiting autophagy. During later phases of OSCC, autophagy reduces Toll-like receptor 4 (TLR4)-dependent EMT. Therefore, inhibition of autophagy by CCL7 may promote TLR4-depedent EMT. Finally, during the advanced stages of OSCC, receptor activator of nuclear factor κB ligand (RANKL), which induces autophagy in OSCC cells, is released from OSCC cells transforming tumor-associated macrophages, TAMs, into osteoclasts, ultimately inducing metastasis.