FIGURE 2.
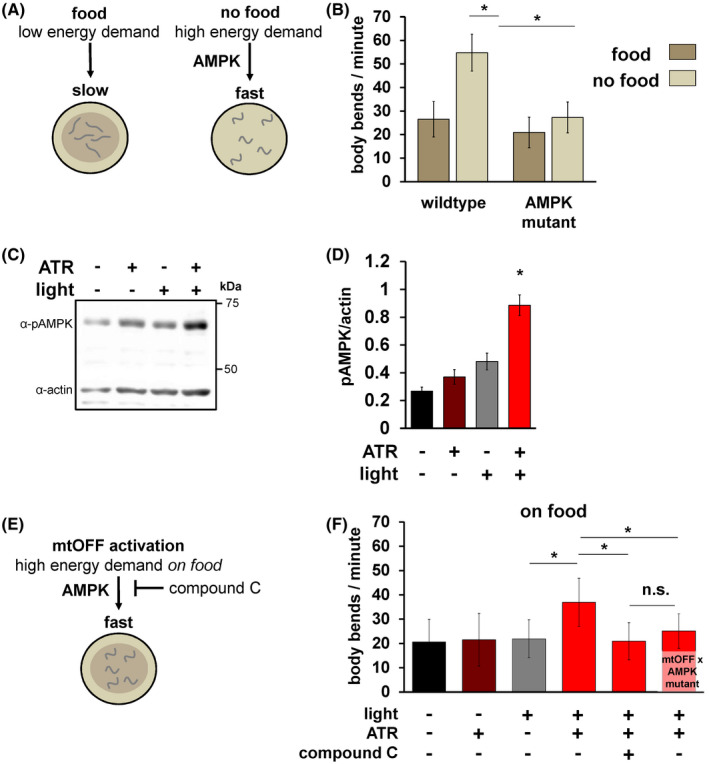
mtOFF modulates energy‐sensing behavior through AMPK. A, Schematic showing locomotion differences in C. elegans under both fed (left) and starved (right) conditions. Removal from food results in increased locomotion, mediated by AMPK signaling. This output is used in this study to validate functional AMPK signaling. B, Locomotion was scored by counting body bends per minute. Animals were scored either on food or immediately after being transferred off of food. C. elegans AMPK is encoded by the aak‐2 gene. The non‐functional aak‐2(ok524) mutant strain was used for AMPK mutant animals. One‐way ANOVA with Tukey's test for multiple comparisons was performed, wild type on food vs wild‐type off of food *P < .0001, wild‐type off of food vs AMPK mutant off of food *P < .0001,. Data are means ± standard deviation, n = 30‐60 animals each condition from at least two experimental days. C, Immunoblot against phosphorylated (active) AMPK (pAMPK, top bands, 62 kDa) and actin (bottom bands, 43 kDa) from whole animal lysate on the same blot shows increased phosphorylation level under conditions of activated mtOFF. D, Quantification of pAMPK/actin densitometry shows increased pAMPK in response to mtOFF activation. pAMPK/actin is used to measure activated AMPK as there is no validated total AMPK antibody in C. elegans. One‐way ANOVA with Tukey's test for multiple comparisons was performed, ‐ATR ‐light vs + ATR +light *P = .0001, +ATR ‐light vs + ATR +light *P = .0006, ‐ATR + light vs + ATR +light *P = .0043. Data are means ± SEM, n = 4 independent blots from one plate of worms for each condition per replicate, with at least 1000 animals per plate. E, Schematic showing effects of mtOFF activation on locomotion. mtOFF is expected to create an energy demand through PMF dissipation that will increase locomotion, mediated by AMPK signaling. F, Body bends were scored, and illumination was throughout measurement where indicated. Animals were exposed to 50 μM compound C for 24 hours where indicated. One‐way ANOVA with Tukey's test for multiple comparisons was performed, −ATR + light vs + ATR +light *P < .0001, +ATR + light vs + ATR +light + compound C *P < .0001, +ATR + light vs mtOFF × AMPK mutant + ATR +light + compound C *P < .0001. n.s. is not significant, P = .3999. Data are means ± standard deviation, n = 30‐60 animals each condition from at least two experimental days
