Figure 17.
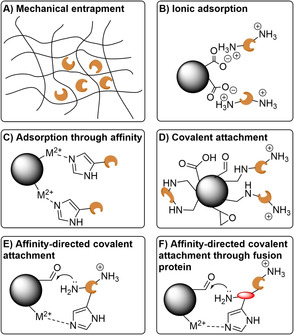
Examples of enzyme immobilization strategies. A) Mechanical entrapment restricts the diffusion of the enzyme. B) Adsorption through ionic interactions, offering little control over the orientation of the enzyme. C) Adsorption though affinity, in this case His‐tag‐metal coordination, allowing control of over the orientation of the enzyme through the tag placement. D) Covalent attachment, offering little control over the orientation of the enzyme. Multipoint attachment can lead to irreversible deformation of the enzyme shape. Common functional groups for covalent attachment are carboxylic acids, aldehydes, and epoxides – using amide formation, reductive amination, and ring opening, respectively. E) Affinity‐directed covalent immobilization orients the enzyme prior to covalent attachment. F) By fusing a (small) protein to the enzyme, covalent immobilization and any shape disruption can be localized to that fusion protein, reducing the effect on the enzyme. However, such an enzyme is more exposed to the environment and stability benefits from immobilization may be diminished. Not shown are covalent crosslinking of enzymes (e. g. using a dialdehyde), and the inherent different properties of supports, with respect to e. g. their size, pore‐size, hydrophilicity/hydrophobicity, etc.
