Figure 2.
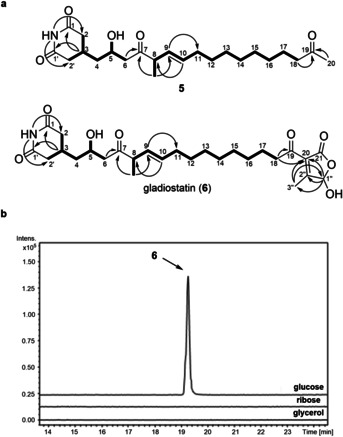
Identification and structure elucidation of metabolic products of the cryptic trans‐AT PKS in B. gladioli BCC1622. a) Structure elucidation of degradation product (5) (top) and gladiostatin (6) (bottom). COSY and key HMBC correlations observed for each compound are indicated by bold lines and arrows, respectively. b) Extracted ion chromatograms at m/z=506.27±0.02, (corresponding to the [M+H]+ ion of gladiostatin) from UHPLC‐ESI‐Q‐TOF‐MS analysis of ethyl acetate extracts from B. gladioli BCC1622 grown on a minimal medium containing different carbon sources.
