Abstract
Background
The goal of this review was to present an overview of the currently identified molecular parameters in head and neck squamous cell carcinoma (HNSCC) of nonsmokers and nondrinkers (NSND).
Methods
Following the PRISMA guidelines, a systematic search was performed using the electronic databases PubMed, Embase, and Google Scholar.
Results
Of the 902 analyzed unique studies, 74 were included in a quantitative synthesis and 24 in a meta‐analysis. Human papillomavirus (HPV) was reported as a molecular parameter in 38 studies, followed by p16 and TP53 (23 and 14 studies, respectively). The variety of other molecular parameters concerned sporadic findings in small numbers of NSND.
Conclusions
HNSCC in NSND is more often related to HPV and p16 overexpression compared to tumors of smokers‐drinkers. In a third of virus‐negative tumors, TP53 mutations were detected with a mutational profile associated with aging and ultraviolet light exposure rather than to tobacco consumption.
Keywords: head and neck cancer, human papillomavirus, nonsmokers, p16, TP53
1. BACKGROUND
Head and neck squamous cell carcinoma (HNSCC) usually results from excessive tobacco and alcohol consumption. 1 A third risk factor in head and neck carcinogenesis is high‐risk human papillomavirus (HPV), especially in the oropharynx. 2 , 3 Patients with HPV‐positive oropharyngeal squamous cell carcinoma (OPSCC) usually have a healthier lifestyle without excessive consumption of tobacco and alcohol compared to patients with HPV‐negative tumors. 4 Additionally, there are HNSCC patients without any exposure to tobacco and alcohol. These nonsmokers and nondrinkers (NSND) appear to be clinically different from their smoking and drinking counterparts: predominantly females at the extremes of age with an early tumor stage, mainly in the oral cavity. 5 , 6 , 7 , 8 , 9 , 10 , 11 Although these clinical differences have been identified, it is partially unclear what starts the carcinogenesis in this group.
In the past decades, the prevalence rate of HPV in HNSCC has been rising in the United States and Europe and many studies have shown that HPV status is a strong, independent prognostic factor for disease free and overall survival in OPSCC. 12 , 13 , 14 Recently, this has led to a down staging of HPV‐positive OPSCC in the Eighth Edition of the American Joint Committee on Cancer and Union for International Cancer Control tumor‐node‐metastasis classification. 15 , 16 An association between HPV positivity and NSND has been suggested in several studies. 17 , 18 , 19
Research into the molecular landscape of HNSCC has increased rapidly in recent years, mainly focusing on differences between these HPV‐positive and HPV‐negative tumors. 3 , 20 In addition to new insights into head and neck carcinogenesis, including its intrinsically immunosuppressive nature, this research has revealed other prognostic biomarkers, diagnostic biomarkers, and targets for novel therapeutic options. 3 , 20 , 21 , 22 , 23 In this field of molecular research, however, little attention has been paid to processes underlying carcinogenesis in NSND. In this systematic review, an overview of the molecular parameters reported in HNSCC of NSND is presented, including a meta‐analysis on the prevalence of HPV, p16 overexpression, and TP53 mutations in NSND vs smokers and drinkers (SD).
2. METHODS
2.1. Search strategy
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses. 24 A systematic search strategy was developed using the electronic databases PubMed, Embase, and Google Scholar combining terms for (a) the head and neck region, (b) squamous cell carcinoma, (c) molecular parameters underlying carcinogenesis, and (d) NSND (Supplementary Table 1). The entire search was performed on October 9, 2018.
2.2. Screening
After discarding duplicate articles using EndNote X7.5 (Clarivate Analytics, Philadelphia, Pennsylvania), two independent reviewers (FM, DP) made the first preselecting cut by screening all articles on title and abstract. Inclusion criteria were as follows: (a) original studies on a (b) viral, protein, or genomic parameter (c) in HNSCC, with (d) results on nonsmokers and/or nondrinkers explicitly reported in the title or abstract, (e) published after 1990. Exclusion criteria were as follows: (a) studies in languages other than English, Dutch, or German, (b) data based on animal samples, (c) skin tumors or rare histological variants of HNSCC, and (d) the gray literature >2 years old. After the first selection, the remaining full‐text articles were assessed for eligibility based on the same criteria. Reference lists of included studies and recent systematic reviews on biomarkers in HNSCC were screened for additional literature. 25 , 26 If an article was not electronically available, the authors were contacted to obtain the full‐text.
2.3. Data extraction and assessment of study quality
For relevant articles, the name of the first author, year of publication, country of conducted research, name of the molecular parameter, tumor location, number of NSND, definition of NSND, study design and method, definition of molecular parameter positivity, and study remarks on the NSND population were retrieved. When at least five articles described the same molecular parameter in NSND, the two reviewers assessed them on methodological quality using a modified 10‐item critical appraisal tool derived from the REporting recommendations for tumor MARKer prognostic studies (REMARK). 27 The critical appraisal criteria were scored with “yes,” “unclear,” or “no” (Supplementary Table 2). External validity was rated with items 1 to 3, and internal validity with items 4 to 10. Dissonance between the two reviewers was dissolved by discussion.
Data were pooled in a meta‐analysis when (a) a clear and acceptable cutoff value for molecular parameter positivity was reported (as was assessed with items 5, 8, and 10 of the quality assessment), and (b) the number of patients positive and negative for the molecular parameter in both NSND and SD was explicitly reported. Studies reporting that these molecular parameters play no role in the head and neck carcinogenesis of NSND were also included to limit selection and publication bias.
2.4. Statistical analysis
Interobserver agreement between the two reviewers for title and abstract screening and full‐text evaluation was determined using Cohen's Kappa coefficient (к). For the meta‐analysis, Review Manager 5.3 (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen) was used to create forest plots by pooling weighted data, calculating the odds ratio (OR) and 95% confidence intervals (CIs) for a fixed effect of molecular parameter presence in NSND using the Mantel‐Haenszel test. To evaluate the statistical reliability of the data, a sensitivity analysis was performed by only retaining studies in the meta‐analysis with at least 10 patients in both the nonsmokers/nondrinkers and smokers/drinkers groups. In case this did not change the outcome, the smaller studies remained included in the meta‐analysis. The I 2 statistic was used for heterogeneity estimation of OR variance between studies. Higgins and colleagues proposed adjectives of low, moderate, and high heterogeneity for I 2 values of 25%, 50%, and 75%, respectively. 28 Since tumor protein p16 overexpression is a surrogate marker for the HPV status in OPSCC, but not in nonoropharyngeal HNSCC (non‐OPSCC), the presence of HPV and p16 overexpression were analyzed separately for OPSCC and non‐OPSCC.
3. RESULTS
3.1. Screening and data extraction
A total of 1039 articles were identified through the electronic search and 7 additional studies from reference lists. After removing duplicates, 902 studies remained for title and abstract evaluation by the two reviewers (к = 0.90 for title and abstract inclusion), 96 of which the full texts were read (к = 0.88 for full‐text inclusion). Seventy‐four studies were included in the qualitative synthesis (Figure 1).
FIGURE 1.
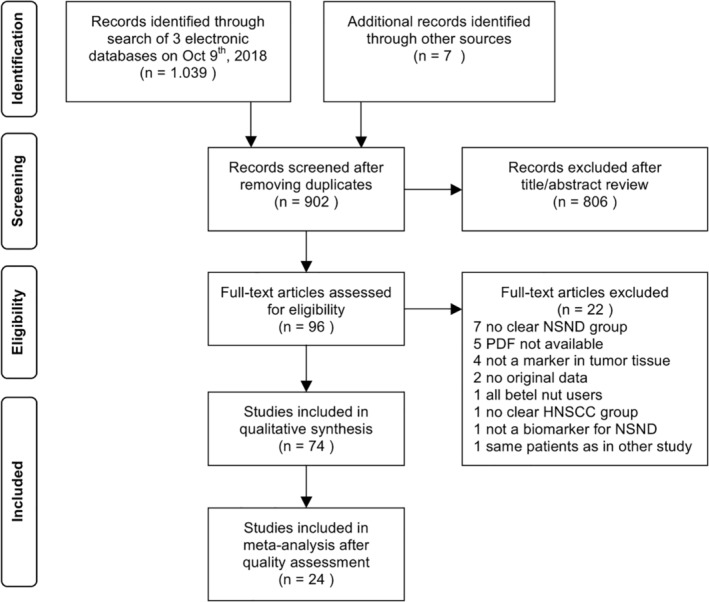
PRISMA flowchart of the literature search. HNSCC, head and neck squamous cell carcinoma; NSND, nonsmokers and nondrinkers
Most studies were published between 2014 and 2018 (58%; 43/74), with the oldest included study being published in 1991. 29 Thirty‐nine percent (29/74) of the publications originated from European institutions, 27% (20/74) from North America, 22% (16/74) from Asia, 8% (6/74) from Central‐South America, and 4% (3/74) from Australia. Half of the included studies (38/74) reported on HPV in nonsmokers and/or nondrinkers, in OPSCC (33%) as well as most other subsites of HNSCC: the oral cavity, hypopharynx, and larynx. Two out of the six studies looking specifically at oral tongue squamous cell carcinoma (OTSCC) found HPV DNA in these tumors using polymerase chain reaction (PCR), and one of these two studies also used real‐time nucleic acid sequence‐based amplification. 30 , 31 The second most frequently evaluated molecular parameter was tumor protein p16, often used as a surrogate marker for HPV infection. TP53 mutations, usually present in OTSCC, and p53 protein expression were analyzed in 19% and 10% of the included studies, respectively (Table 1). Although a variety of other molecular parameters have been reported, these concerned sporadic findings and were mostly identified in small numbers of NSND (Figure 2). However, most noticeable were the number of studies indicating a higher impact of the immune response in tumors of NSND compared to SD, with the description of the interferon γ (INFγ) and nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NFKB) pathways, including interleukin‐10 (IL‐10), programmed death‐1 (PD‐1), programmed death‐ligand 1 (PD‐L1), indoleamine 2,3‐dioxygenase 1 (IDO‐1), and tumor‐infiltrating lymphocytes (TILs) (Supplementary Table 3).
TABLE 1.
HPV, p16, p53, and TP53 in head and neck squamous cell carcinoma of nonsmokers and nondrinkers
| Reference (country) | Molecular parameter | Tumor location | Number of patients (positive cases) | Definition NSND | Study remarks | |
|---|---|---|---|---|---|---|
| NS | ND | |||||
| HPV | ||||||
| Amsbaugh et al 32 (United States) | HPV combined with p16 | Oropharynx | 79 (NA) | — | NS = never smoking | The rates of HPV/p16 positivity, never smoking, and cervical lymph node metastases were significantly higher for patients with OPSCC of the tonsil, base of tongue, or vallecula subsites when compared with pharyngeal wall or palate subsites |
| Andrews et al 17 (United States) | HPV | Oropharynx | 18 (14) | 18 (14) | NSND = no prior or current use of tobacco and/or alcohol | HR‐HPV infection is a predominant risk factor in the development of OPSCC in patients who do not smoke or drink |
| Ang et al 33 (United States) | HPV | Oropharynx | 73 (59) | — | NS = never smoked | HPV‐positive oropharyngeal cancer was more common among patients who had never smoked |
| Angiero et al 34 (Italy) | HPV | Oral cavity | 11 (3) | 11 (3) | NSND = nonsmoker and nondrinker patients | The presence of HPV DNA appeared to be a molecular marker in dysplasia and OSCC of a subgroup of nonsmoker and nondrinker patients |
| Antonsson et al 35 (Australia) | HPV |
Head and neck Oropharynx |
19 (0) 8 (7) |
20 (2) 6 (5) |
NSND = self‐reported lifelong nonsmoker, nondrinker | HPV prevalence and p16 overexpression were highest in OPSCC, younger patients, and nonsmokers |
| Bragelmann et al 36 (United States) | Viral mRNA | Oral tongue | 7 (0) | 7 (0) |
NS < 5PY ND ≤ 1 glass of wine or equivalent/day |
None of the seven OTSCC showed significant presence of viral transcripts |
| Chen et al 37 (China) | HPV | Oral cavity | 89 (NA) | 105 (NA) |
NS < 100 cig/lifetime ND < 1 drink/week for at least 6 months |
Oral HPV infection was strongly associated with an increased risk of OSCC in females, young adults, married population, merchants, nonsmokers, nonalcohol drinkers, and nontea drinkers |
| Chen et al 38 (Taiwan) | HPV | Larynx | 13 (4) | 55 (10) | — | Patients with HPV‐positive tumors were older, less local/regional recurrence, and nonsmoker. A low prevalence of HPV infection in our series suggests that HPV is not a major cause of LSCC |
| Chen et al 39 (China) | HPV | Larynx | 70 (13) | 110 (12) |
NS = never smoking ND = never drinking |
The risk of LSCC associated with HPV‐16 DNA positivity was even higher in patients aged 55 years or younger, males, never smokers, and never drinkers |
| Chuang et al 40 (China) | HPV | Oral cavity | 73 (53) | 99 (64) | — | HPV‐16/18 infection rates in females, nonsmokers, nondrinkers, and nonbetel quid chewers were higher than in males, smokers, drinkers, and betel quid chewers |
| Dediol et al 41 (Croatia) | HPV | Oral cavity | 77 (17) | 77 (17) |
NS < 10PY ND = no alcohol on daily basis |
In contrast to OPSCC, HPV in OSCC is a negative predictive factors [for disease‐specific survival], especially in NSND patients |
| Descamps et al 42 (Belgium) | HPV | Head and neck | 24 (6) | 50 (12) | NSND = never used tobacco or alcohol | We observed a significantly worse prognosis for consumers of alcohol and tobacco compared to nondrinkers and nonsmokers |
| Farnebo et al 43 (Sweden) | HPV | Head and neck | 26 (20) | — | NS = never smoker | HPV‐positive never smokers had lower frequencies of TP53 mutations. |
| Farshadpour et al 18 (Netherlands) | HPV combined with p16 | Oropharynx | 16 (12) | 16 (12) | NSND = no history of smoking tobacco and alcohol consumption | All HPV‐positive tumors showed p16 overexpression. HPV is strongly associated with OPSCC of nonsmoking and nondrinking patients |
| Fouret et al 44 (France) | HPV | Head and neck | 10 (5) | — | NS = 0PY | HPV may play a role in HNSCC in nonsmokers |
| Gillison et al 45 (United States) | HPV | Head and neck | 23 (16) | 23 (16) |
NS < 1 cig/day for a year ND < 1 alcoholic drink/day for a year |
Compared with subjects who neither smoked tobacco nor drank alcohol, those with heavy use of tobacco and alcohol had an increased risk of HPV‐16‐negative HNSCC |
| Gonzalez‐Ramirez et al 46 (Mexico) | HPV | Oral cavity | 42 (4) | 47 (4) | NSND = no current or former tobacco or alcohol use | All HR‐HPV‐positive OSCC cases corresponded to young patients, nonsmokers, and nonalcohol drinkers |
| Hafkamp et al 47 (Netherlands) | HPV | Oropharynx | 12 (10) | 31 (18) |
NS = never smoker or former smoker >10 years before SCC ND ≤2 whiskey equivalents/day |
The presence of HPV‐16 proved to be a strong independent predictor of favorable outcome in nonsmokers |
| Hoffmann et al 48 (Germany) | HPV combined with p16 | Oropharynx | 36 (NA) | — | NS ≤10PY | Nonsmoking HPV‐positive TSCC patients show 10‐year OS of 100% and 90.9% PFS when treated with adjuvant RCT |
| Hong et al 49 (Australia) | HPV combined with p16 | Oropharynx | 73 (59) | 53 (32) |
NS = nonsmoker ND = nondrinker |
Our data show a rising prevalence of HPV‐positive OPSCC in Australia over the last two decades, with patients presenting at an older age and about one third have never smoked |
| Joo et al 50 (Korea) | HPV | Hypopharynx | 18 (5) | 28 (5) |
NS = never smoked ND = nondrinkers |
Significant correlations were found between positive HR‐HPV and younger age and nonsmoking status |
| Laco et al 19 (Czech Republic) | HPV |
Oral cavity Oropharynx |
24 (3) 22 (18) |
24 (3) 22 (18) |
NSND = no history of either smoking or chronic alcohol abuse | The majority of tumors developing in patients with OPSCC without positive personal history of smoking and alcohol abuse are related to oral HPV infection, whereas the viral etiology is responsible for a substantially smaller subset of OSCC |
| Li et al 51 (United States) | HPV | Oral tongue | 6 (0) | — | NS = no history of tobacco smoking or chewing | No HPV was found in any of the tumors other than the HPV‐positive control |
| Maruyama et al 52 (Japan) | HPV | Oropharynx | 22 (13) | 37 (20) |
NS ≤5PY ND < 5 units of sake/day for a year |
In OPSCC, which showed an increasing trend of HPV prevalence over time, HPV infection was inversely correlated with tobacco smoking, alcohol drinking, TP53 mutations, and a disruptive [gene] mutation |
| Mena et al 53 (Spain) | HPV combined with p16 | Oropharynx | 82 (29) | 137 (32) |
NS = nonsmoker ND = nondrinker |
Being non‐smoker or nondrinker was consistently associated across HPV‐relatedness definitions with HPV positivity |
| Oliveira et al 31 (Brazil) | HPV | Oral cavity | 16 (7) | — | NS = never smoked | The tongue was the most prevalent infected anatomical site. A significant number of HPV samples were positive among nonsmoking patients |
| Peterson et al 54 (United States) | HPV | Head and neck | 96 (52) | 67 (NA) | NS = self‐reported never smoker | In HPV‐positive patients, for overall, recurrence‐free, and disease‐specific survival, nonsmokers showed marginal improvements in survival compared to smokers |
| Platek et al 55 (United States) | HPV | Oropharynx | 26 (22) | — | NS = never smoker | When HPV status was stratified by smoking status, the OS favored never/former smokers vs current smokers, but the difference only reached statistical significance for patients with HPV‐positive tumors |
| Poling et al 56 (United States) | HPV | Oral tongue | 44 (0) | 57 (0) |
NS = never tobacco on regular basis ND < 10 units/week |
HPV E6/E7 mRNA transcripts were detected in only 1 [smoker] case |
| Quabius et al 57 (Germany) | HPV | Head and neck | 60 (31) | — | NS = nonsmoker | The surplus of annexin A2 in nonsmokers and HPV‐positive patients supports our hypothesis that decreased SLPI levels facilitate HPV infection |
| Schlecht et al 58 (United States) | HPV | Head and neck | 7 (3) | 21 (7) |
NS = nonsmoker ND = light drinker <4 drinks/week for 3 years |
Focusing on never smokers, we identified a distinct subset of 123 genes that were specifically dysregulated in HPV16‐positive HNSCC |
| Siebers et al 59 (Netherlands) | HPV | Oral tongue | 7 (0) | 7 (0) |
NS = never smoked ND ≤1 unit alcohol/day |
No HPV was detected in these specimens. |
| Simonato et al 60 (Brazil) | HPV | Oral cavity | 3 (2) | 11 (3) |
NS = no tobacco consumption ND = no alcohol consumption |
The highest prevalence of HPV DNA was observed in nonsmoking patients over the age of 60 years. |
| Tachezy et al 61 (Czech Republic) | HPV | Oral cavity/ oropharynx | 7 (7) | 16 (11) |
NS = smoked <0.5 pack/week for a year ND < 1 drink/week for a year |
The prevalence of HPV DNA was lower in OSCC than in OPSCC, and higher in NSND. |
| Tsimplaki et al 30 (Greece) | HPV | Oral tongue | 15 (5) | 15 (5) | NSND = no tobacco and no alcohol use | HPV infection was strongly associated with abstinence from tobacco and alcohol. |
| Vatca et al 62 (United States) | HPV | Oropharynx | 42 (38) | — | NS = stopped >1 year before diagnosis and < 10 PY | Risk factors for OPSCC modify the incidence of treatment‐related early toxicities, with HPV‐positive and nonsmoking status correlating with increased risk of high‐grade mucositis |
| Wangsa et al 63 (United States) | HPV | Oral tongue | 20 (0) | — | NS = not smoking | The one patient that tested positive for HPV‐16 was a Stage 4 patient that smoked |
| Xu et al 64 (China) | HPV combined with p16 | Larynx | 115 (12) | 236 (17) |
NS = smoking < once/week ≥1 year ND < 1 unit alcohol/week ≥1 year |
HPV infection was more common among nonsmokers, nondrinkers, and patients with supraglottic LSCC. |
| p16 | ||||||
| Angiero et al 34 (Italy) | p16 | Oral cavity | 11 (6) | 11 (6) | — | No specific remark regarding p16 and nonsmokers and/or nondrinkers |
| Antonsson et al 35 (Australia) | p16 | Head & neck | 26 (8) | 24 (8) | NSND = self‐reported lifelong nonsmoker, nondrinker | p16 overexpression was highest in OPSCC, younger patients, and nonsmokers. |
| Dediol et al 41 (Croatia) | p16 | Oral cavity | 77 (21) | 77 (21) | — | In contrast to OPSCC, p16 expression in OSCC is a negative predictive factor [for disease specific survival], especially in NSND patients. |
| Gillison et al 65 (United States) | p16 | Oropharynx | 96 (81) | — | NS = never smoker | p16 positive patients were more likely to be never smokers and had significantly lower cigarette smoking exposure. |
| Haas et al 66 (Germany) | p16 | Oropharynx | 24 (7) | 24 (7) |
NS < 10PY ND = no alcohol on daily basis |
p16 was the only marker showing a significant correlation with a negative smoking history. |
| Habbous et al 4 (Canada) | p16 | Oropharynx | 755 (NA) | 2032 (NA) |
NS = never smoked ND = no/light alcohol consumption (≤ 2 drinks/day) |
Variables associated with p16‐positive status are male sex, tonsillar or base‐of‐tongue tumors, smaller tumors, nodal involvement, less smoking and lower alcohol consumption. |
| Heaton et al 67 (United States) | p16 | Oral tongue | 50 (5) | — | NS < 100 cig in lifetime | There was no correlation found between p53 and p16 IHC status and the clinicopathologic variables studied. |
| Hess et al 68 (United States) | p16 | Oropharynx | 66 (60) | 30 (24) |
NS = never smokers ND = self‐reported rare alcohol use |
Self‐reported heavy alcohol use was significantly higher among p16‐negative patients and more p16‐positive patients identified themselves as “never smokers” |
| Kalfert et al 69 (Czech Republic) | p16 | Larynx | 8 (6) | — | NS = nonsmoker | p16 expression in glottic LSCC, especially in subgroup of nonsmokers, might be a promising prognosticator of better clinical outcome in routine practice. |
| Karpathiou et al 70 (France) | p16 | Head & neck | 6 (4) | — | NS = not smoking | p16 positivity and p53 normal expression were significantly correlated with nonsmoking, an earlier T stage and a nonkeratinizing morphology. |
| Laco et al 19 (Czech Republic) | p16 |
Oral cavity Oropharynx |
24 (7) 22 (22) |
24 (7) 22 (22) |
NSND = no history of either smoking or chronic alcohol abuse | In this population of NSND, p16 expression was detected in 29% of OSCC and 100% of OPSCC |
| Mafune et al 71 (Japan) | p16 | Head & neck | 35 (2) | — |
NS ≤10PY prior to surgery or stopped ≥20 years ND < 1 drink/day |
Nonsmokers did not differ significantly from smokers with regard to p16 |
| Poling et al 56 (United States) | p16 | Oral tongue | 44 (5) | 57 (5) |
NS = never tobacco on regular basis ND < 10 units/week |
p16 overexpression was detected in 9 of 78 cases |
| Ralli et al 72 (India) | p16 | Head & neck | 10 (7) | 29 (21) | — | Expression of p16 was higher in nonsmokers and nonalcohol consumers and significantly associated with paan chewing habit. |
| Silva et al 73 (Brazil) | p16 | Oropharynx/ larynx | 4 (4) | 13 (7) |
NS = no smoking habit ND = no alcohol consumption |
p16 expression was more intense in nonsmoking patients, whose tumors showed negative vascular embolization, negative lymphatic permeation, and clear surgical margins. |
| Ye et al 74 (Canada) | p16 | Oropharynx | 52 (45) | — | NS = never smoker | Most patients were p16‐positive, were younger (predominantly male), mostly former or nonsmokers, and had a more advanced nodal stage. |
| Zhao et al 75 (United States) | p16 | Oropharynx | 5 (2) | — | NSND = never smoker never drinker | Different p16 protein localization suggested different survival outcomes in a manner that does not require limiting the biomarker to the oropharynx and does not require assessment of smoking status |
| p53 | ||||||
| Angiero et al 34 (Italy) | p53 | Oral cavity | 11 (9) | 11 (9) | — | No specific remark regarding p53 and nonsmokers and/or nondrinkers |
| Fernandez‐Acenero et al 76 (Spain) | p53 | Larynx | 21 (8) | 21 (8) | NSND = never smoked or drank alcohol | p53 expression seems to negatively influence survival in nonsmoking nonalcoholic patients with LSCC. |
| Field et al 29 (United Kingdom) | p53 | Head & neck | 7 (1) | — | NS = nonsmoker | Six out of seven nonsmokers did not express p53 whereas 29 of 37 heavy smokers were found to have elevated p53 expression |
| Haas et al 66 (Germany) | p53 | Head & neck | 24 (17) | 24 (17) | NSND = never used tobacco or alcohol on a regular basis | Expression of p53 was independent of smoking history and tumor site |
| Heaton et al 67 (United States) | p53 | Oral tongue | 51 (16) | — | NS < 100 cig in lifetime | There was no correlation found between p53 and p16 IHC status and the clinicopathologic variables studied |
| Karpathiou et al 70 (France) | p53 | Head & neck | 6 (3) | — | NS = not smoking | p16 positivity and p53 normal expression were significantly correlated with nonsmoking, an earlier T stage and a nonkeratinizing morphology |
| Matthews et al 77 (Netherlands) | p53 | Oral tongue | 14 (7) | 9 (6) | NSND = nonsmokers nondrinkers | There was an apparent negative association between IHC detection of p53 and tobacco smoking and/or alcohol intake |
| TP53 | ||||||
| Faden et al 78 (United States) | TP53 | Oral tongue | 43 (NA) | — | NS = never smoker | OTSCC in nonsmokers have TP53 mutation rates similar to other HNSCC, yet these mutations do not appear related to carcinogen exposure based on the mutational spectrum and clinical history |
| Farnebo et al 43 (Sweden) | TP53 | Head & neck | 7 (3) | — | NS = never smoker | HPV‐positive never smokers had lower frequencies of TP53 mutation |
| Fouret et al 44 (France) | TP53 | Head & neck | 10 (0) | 0 | NS = 0PY | There were no TP53 gene mutations in cancer cells |
| Heaton et al 67 (United States) | TP53 | Oral tongue | 47 (10) | — | NS < 100 cig in lifetime | TP53 and CDKN2a mutations in never‐smoker OTSCC are associated with worse clinicopathologic characteristics and poorer survival outcomes |
| Hong et al 79 (Australia) | TP53 | Oropharynx | 33 (10) | 38 (14) |
NS = never smoker ND = never drinker |
Among patients with HPV‐positive OPSCC, there was no significant difference in TP53 mutation by smoking status. HPV‐positive OPSCC are less likely to have mutant TP53 than HPV‐negative OPSCC |
| Li et al 51 (United States) | Gene mutations, including TP53 | Oral tongue | 6 (1) | — | NS = no history of tobacco smoking or chewing | The recurrently mutated genes in our cohort of cancers from nonsmokers were CTNNA3, EIF3A, EP300, FXR1, NEK8, NOTCH1, PIK3CA, PKHD1L1, PTCHD2, RALGAPB, SPEN, and UBR4. Nonsmokers had fewer TP53 mutations than smokers |
| Mafune et al 71 (Japan) | TP53 | Head and neck | 71 (37) | 89 (50) |
NS ≤10PY prior to surgery or stopped ≥20 years ND < 1 drink/day |
In nonsmokers, 24% of TP53 mutations occurred at CpG sites, but in smokers, 12% did |
| Maruyama et al 52 (Japan) | TP53 | Head & neck | 47 (15) | 59 (14) |
NS ≤5PY ND < 5 units of sake/day for a year |
In OPSCC, HPV infection was inversely correlated with tobacco smoking, alcohol drinking, TP53 mutations, and a disruptive [gene] mutation |
| Mirghani et al 80 (France) | Mutation profiles, including TP53 | Oropharynx | 37 (3) | — | NS = without any smoking history | Mutation rate [of TP53] was not significantly different in smokers compared with nonsmokers, even when analyses focused on heavy smokers |
| Ostwald et al 81 (Germany) | TP53 | Oral cavity | 23 (10) | 26 (9) | NSND = no history of smoking or drinking | The rate of lip tumors with mutations was higher in nonsmokers than in smokers. In contrast, TP53 mutations in intraoral tumors clustered in smokers |
| Pickering et al 82 (United States) | Gene mutation frequencies, including TP53 | Oral tongue | 44 (31) | — | NS < 1PY smoking history | Three genes showed trends toward statistical significance: FAT1, TP53, and PIK3CA. However, not between the younger and older patient cohorts |
| Tan et al 83 (Singapore) | Mutation profiles, including TP53 | Oral tongue | 25 (3) | — | NS = never smoker | There was no significant association between smoking history and the presence of any mutation detected by the LungCarta panel, or specific alterations in MET, TP53, and STK11 |
| Wangsa et al 63 (United States) | FISH markers, including TP53 | Oral tongue | 20 (NA) | — | NS = not smoking | Copy number increases of all five markers were found to be correlated to nonsmoking habits, while smokers in this cohort had low‐level copy number gains |
| Zanaruddin et al 84 (Malaysia) | TP53 | Oral cavity | 24 (8) | 24 (8) | NSND = no smoking, alcohol or chewing habit | TP53 truncating mutations were more common in patients with no risk habits |
Abbreviations: cig, cigarettes; HNSCC, head and neck squamous cell carcinoma; HPV, human papilloma virus; HR, high risk; IHC, immunohistochemistry; LSCC, laryngeal squamous cell carcinoma; NA, not available; ND, nondrinkers; NS, nonsmokers; OPSCC, oropharyngeal squamous cell carcinoma; OS, overall survival; OSCC, oral squamous cell carcinoma; OTSCC, oral tongue squamous cell carcinoma; PFS, progression‐free survival; PY, pack‐years; RCT, radiochemotherapy; SCC, squamous cell carcinoma; TSCC, tonsillar squamous cell carcinoma.
FIGURE 2.
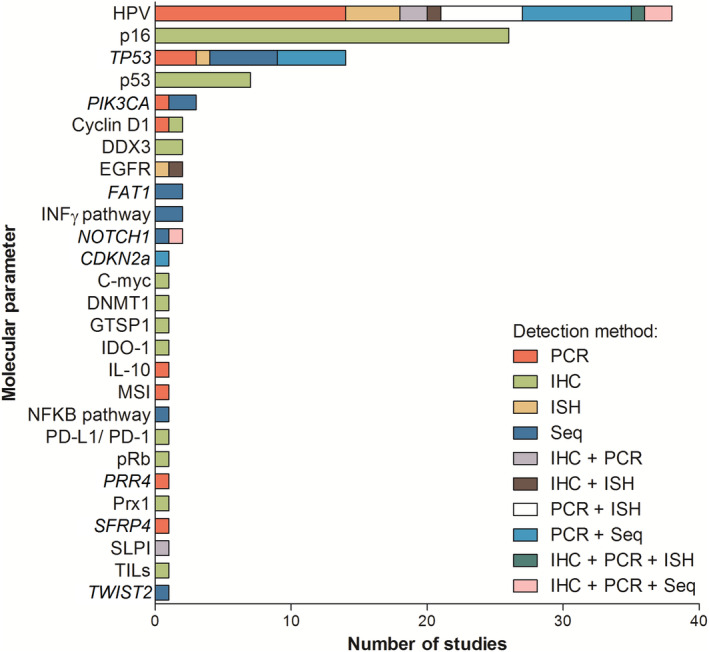
Number of studies and type of detection method of molecular parameter evaluation in head and neck squamous cell carcinoma of nonsmokers and nondrinkers. IHC, immunohistochemistry; ISH, in situ hybridization; PCR, polymerase chain reaction; Seq, sequencing; TILs, tumor‐infiltrating lymphocytes [Color figure can be viewed at wileyonlinelibrary.com]
3.2. Assessment of study quality
Eleven included studies met all criteria for external validity, 37 , 41 , 44 , 45 , 54 , 59 , 61 , 62 , 64 , 67 , 71 whereas another study met all criteria for internal validity. 35 Five out of seven criteria for internal validity were met in 10 studies. 18 , 53 , 58 , 59 , 68 , 73 , 75 , 79 , 80 , 84 Whether or not the molecular parameter was interpreted without knowledge of the patients' clinical characteristics was the most frequently underreported critical appraisal item (20% scored yes), followed by the items univariate and multivariable statistics in particular for NSND (32%) and the item of a clear NSND definition (34%)(Supplementary Table 4).
A molecular parameter in an exclusively NSND population was reported in 14 studies. 17 , 18 , 19 , 30 , 34 , 36 , 41 , 45 , 59 , 76 , 84 , 85 , 86 , 87 For the other studies, it was unclear if the nonsmokers were the same patients as the nondrinkers and vice versa. There was a large variety in definitions for considering someone as a NSND. Usually, it was a general definition like “never used tobacco or alcohol.” More specific definitions for nonsmoking varied from “<100 cigarettes in their lifetime” to “…smoked less than 10 pack‐years prior to the surgical resection of HNSCC.” 67 , 71 For nondrinking, the definitions ranged from never having “consumed at least 1 drink/week continuously for at least 6 months” to “drinking less than five units of sake (=140 g alcohol) per day for 1 year” (Table 1). 37 , 52
3.3. Meta‐analysis on molecular parameters HPV, p16 overexpression, and TP53 mutations
Twelve studies detected HPV presence using at least two identification techniques in HNSCC of nonsmokers, and all but one of these studies reported on nondrinkers too. HPV‐16 was the most frequently detected parameter, followed by HPV‐18 and HPV‐33. Furthermore, HPV types 31, 35, 51, 56, and 58 were described as well, although it was not specified if these types were present in the NSND and/or SD population. HPV was found significantly more frequent in NSND compared to SD (ORnonsmoker = 6.22, 95% CI 4.65‐8.32, P < .001, I 2 = 45%; ORnondrinker = 3.45, 95% CI 2.59‐4.61, P < .001, I 2 = 31%) (Figure 3A,B). This significant difference in prevalence was more pronounced in OPSCC, with a pooled prevalence of 62% (n = 146/237) in nonsmokers and 41% (n = 101/249) in nondrinkers, compared to a HPV prevalence of 21‐22% (n = 284/1.385 and n = 259/1200) in the SD group. In non‐OPSCC, the HPV prevalence was approximately 22% (n = 31/141 and n = 46/224) in NSND vs 11% (n = 79/683 and n = 51/489) in SD.
FIGURE 3.
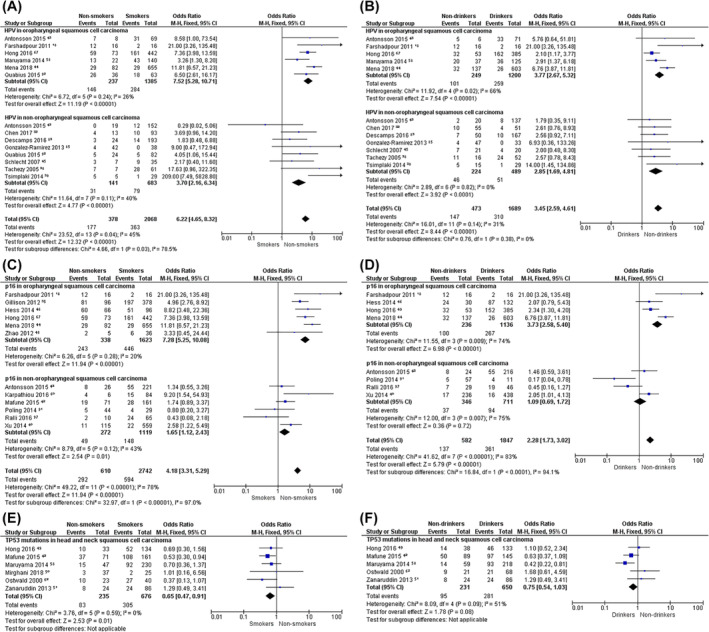
Meta‐analysis on the prevalence of molecular parameters HPV (A,B), p16 overexpression (C,D) and TP53 mutations (E,F) in head and neck squamous cell carcinoma of nonsmokers vs smokers (A,C,E) and nondrinkers vs drinkers (B,D,F). The presence of HPV and p16 overexpression were analyzed separately for oropharyngeal and nonoropharyngeal squamous cell carcinoma [Color figure can be viewed at wileyonlinelibrary.com]
Of the 12 studies describing a strong and diffuse p16‐staining pattern in tumors of nonsmokers, 8 presented data on nondrinkers as well. In OPSCC, p16 overexpression was significantly more prevalent in nonsmokers (OR = 7.28, 95% CI 5.25‐10.08, P < .001, I 2 = 20%) and nondrinkers (OR = 3.73, 95% CI 2.58‐5.40, P < .001, I 2 = 74%) compared to SD. Similar results were found in non‐OPSCC of nonsmokers vs smokers (OR = 1.65, 95% CI 1.12‐2.43, P = .01), which just remained significantly different after sensitivity analysis (OR = 1.51, 95% CI 1.01‐2.26, P = .04) (Supplementary Figure 1), but not in nondrinkers vs drinkers (OR = 1.09, 95% CI 0.69‐1.72, P = .72) (Figure 3C,D).
Tumor protein p53 could not be pooled because definitions for positivity were too heterogeneous, ranging from a “clear brown color, regardless of the staining intensity” and “>5% staining” to “≥50% nuclear/cytoplasmic staining.” 66 , 67 , 70 , 77 When looking at least at exon 5‐8 (coding the DNA binding portion of p53 and containing >90% of the mutations described in HNSCC), TP53 mutations were found in 35% of the 235 nonsmokers presented in the six included studies. 54 Though this percentage is significantly lower than the prevalence of TP53 mutations found in the smokers group of these studies (45% [n = 305/676], OR = 0.65, 95% CI 0.47‐0.91, P = .01), it still is a considerable percentage. When pooling the data on nondrinkers and drinkers, there was no significant difference in TP53 mutation prevalence (OR = 0.75, 95% CI 0.54‐1.03, P = .09), with 41% of the 231 nondrinkers having a TP53 mutation (Figure 3E,F). The TP53 mutations usually consisted of a G:C‐A:T transition, which is a mutational signature related to aging and ultraviolet light exposure. 52 , 81 , 84 , 88 In addition, mutations in the abovementioned studies were reported in exons 4 to 8 and 10, repeatedly at a CpG site, and were less common in nasopharyngeal squamous cell carcinoma and HPV‐positive OPSCC. 52 , 71 , 79 , 80 , 81 , 84
4. DISCUSSION
The rapidly developing field of molecular research is identifying a growing number of biomarkers for cancer diagnosis, prognosis, therapy selection, or therapy effect evaluation. Despite the rich body of molecular data on HNSCC in SD, there is little comprehensive information on specific molecular parameters underlying carcinogenesis in NSND, in which the carcinogenesis is expected to be different. In the reviewed literature, the most prevalent and most frequently reported molecular parameters in NSND are well known from tumors in SD: HPV, tumor protein p16 overexpression, TP53 mutations, and tumor protein p53 immunohistochemistry (IHC). Nonetheless, there is substantial heterogeneity in definitions for both constructs; NSND and parameter positivity. The current meta‐analysis showed a higher prevalence of HPV in both OPSCC and non‐OPSCC of NSND compared to HNSCC in SD. Similar results were found for p16 overexpression in OPSCC of NSND and in non‐OPSCC of nonsmokers. Remarkably, specific TP53 mutations were detected in more than a third of the included NSND.
A great variety in the definition of the construct NSND was found in the literature, even including descriptions such as “less than 10 pack years prior to the surgical resection of HNSCC” or “drinking <140 g alcohol/day for a year.” 52 , 71 The International Head and Neck Cancer Epidemiology (INHANCE) consortium encountered a similar variety in the definition of the construct NSND in their pooled data analysis from patients in Europe and the Americas, with definitions such as “smoking one‐half pack or more per week for ≥1 year” and “consumed an average of one or more drinks per week for 1 or more years” for smokers and drinkers, respectively. 89 For smoking, an accurate definition seems necessary, as the INHANCE consortium concluded that there is no harmless level of tobacco consumption, with already an increased risk of getting HNSCC when smoking >0‐3 cigarettes/day. 90 In their re‐analysis of case‐control studies, Dal Maso and colleagues also found a steep increase in HNSCC risk with increased tobacco consumption, starting from 1 cigarette/day, regardless of ethanol intake. 1 However, for alcohol, there seems to be a threshold effect at approximately 50 g/day in nonsmokers before the increased HNSCC risk starts. 1 Therefore, when analyzing nondrinkers, a less strict definition of the construct nondrinking may be opted for.
The present meta‐analysis showed that the HPV and p16 overexpression prevalence in OPSCC was over 60% (nHPV = 146/237 and np16 = 243/338) in nonsmokers and 40% (nHPV = 101/249 and np16 = 100/236) in nondrinkers, compared to 20% (nHPV = 284/1.385, nHPV = 259/1.200, np16 = 446/1.623, and np16 = 267/1.136) in SD. A wide range of HPV prevalence has been reported in both OPSCC and non‐OPSCC, summarized by Kreimer and colleagues in their systematic review of 60 studies, with an overall HPV prevalence of 36% (n = 345/969) in OPSCC. 91 This is higher than the HPV prevalence in SD of the current meta‐analysis, but HPV status was solely based on PCR results and the smoking and drinking habits of the patients were not reported in the study by Kreimer and colleagues. Our results are in concordance with other studies analyzing large cohorts of OPSCC based on HPV DNA in combination with either E6*I mRNA or p16 IHC, where a HPV prevalence of 22% (n = 243/1.085) was found, rising up to 50% to 60% (patient numbers not displayed) in patients from South America, Northern Europe, Central Eastern Europe, and Australia, and going further up to 80% (n = 59/73) in nonsmokers. 49 , 92 Although the first phase III de‐escalation trial for HPV‐positive OPSCC had turned out in favor of the standard treatment cisplatin‐based (opposed to cetuximab‐based) chemoradiotherapy, including >50% nonsmokers (defined as “never smoked”) in both study arms, results of other trials are still being awaited. 93 , 94 Therefore, the higher HPV prevalence in NSND might affect the treatment strategy of these patients considerably.
The present meta‐analysis determined a HPV prevalence just over 20% (n = 31/141 and n = 46/224) in non‐OPSCC of NSND, being comparable to the HPV prevalence in OPSCC of SD (n = 284/1385 and n = 259/1200). The SD with non‐OPSCC had a significantly lower HPV prevalence of 11% (n = 79/683 and n = 51/489). These percentages are higher than Castellsagué and colleagues found in their analysis of oral (n = 1264) and laryngeal (n = 1042) squamous cell carcinoma, with a HPV prevalence up to 7% in South America, Central America, and Northern Europe. 92 This difference might be the result of inclusion of more recent studies in the present systematic review in combination with a worldwide rising HPV prevalence, or because of a higher prevalence in NSND. Kreimer and colleagues reported an overall HPV prevalence in non‐OPSCC similar to the prevalence of the NSND. 91 Again, this might be an overestimation since these data are only based on HPV detection using PCR and on the HPV prevalence including SD.
Contrary to expectations, the p16 overexpression and HPV prevalence were similar, both in OPSCC and non‐OPSCC. Therefore, it has been recommended to combine PCR, ISH, IHC, or sequencing assays for obtaining an optimal sensitivity and specificity for biologically active HPV detection. 53 , 95 , 96 This is clinically relevant because only OPSCC with transcriptionally active HPV are related to a better survival compared to biologically inactive variants. 53 , 96 This difference in sensitivity/specificity between HPV DNA and p16 IHC detection was reported in several studies reviewed in the present meta‐analysis too, with none of the studies presenting a perfect relationship between HPV DNA and p16 IHC detection, neither in OPSCC nor in non‐OPSCC. 18 , 35 , 49 , 53 Therefore, only studies confirming the presence of HPV with at least two techniques were included in this meta‐analysis, with p16 IHC being a valid confirmation technique in OPSCC when there was ≥70% positivity or diffuse intense/strong staining (Supplementary Table 2).
Following genome sequencing data, signatures of TP53 mutational processes in human cancers have previously been determined. 88 , 97 Signatures contributing to a significant number of somatic TP53 mutations in HNSCC include signature 1B (associated with aging), signature 2 (associated with apolipoprotein B editing complex), signature 4 (associated with smoking), and signature 7 (associated with ultraviolet light exposure). Signature 1 is related to relatively elevated rates of spontaneous deamination of 5‐methyl‐cytosine that are acquired over a human lifetime, at a relatively constant rate in normal somatic tissue that is similar in different people, which may result in cancer in elderly people via C > T transitions. 97 This mutation is in concordance with the TP53 G:C‐A:T transitions reported in two of the included studies of this meta‐analysis (C > T in 14% (1/7) and 41% (9/22)). 52 , 84 An explanation for a higher prevalence of this signature could be the typically higher age of NSND compared to SD. 7 , 9 , 10 , 11 Signature 7 shows a higher prevalence of C > T mutations in untranscribed strands of genes following ultraviolet light exposure, impairing the transcription‐coupled nucleotide excision repair. This fits the C > T mutations found in lip tumors of one included study (C > T in 60% [6/10]), where sunlight might play a dominant role in squamous cell carcinoma of the lip area between the vermilion border and wet line. 81 Although C > A mutations, typical for smoking‐related tumors as a result of the tobacco carcinogen benzo[α]pyrene, have previously been observed in smaller numbers of oral cavity and pharyngeal tumors of nonsmokers, this finding could not be confirmed in the current meta‐analysis. 88 These data strengthen the premise of a different pathway of carcinogenesis resulting in TP53 mutations in HNSCC of NSND compared to SD, with a more prominent role of spontaneous C > T mutations acquired over a patient's lifetime as a result of aging in the former group, opposed to C > A mutations resulting from tobacco exposure in the latter group.
TP53 mutations are of interest as a biomarker because tumors containing these are associated with a more aggressive and therapy‐resistant phenotype. 98 , 99 Many studies analyzed the concordance between TP53 mutations and its gene product, p53 protein expression, as a cheaper and faster IHC assay. 100 , 101 In addition, p53 activity is often inactivated following the expression of oncoprotein E6. 34 However, discrepancies have been reported between p53 IHC and the mutational status of the TP53 gene. 100 , 101 Possible explanations proposed by Hafkamp and colleagues include the following: (a) the frequently used IHC DO‐7 antibody binds to both normal and mutant p53 protein, (b) the TP53 mutations occur outside the common exons 5 to 8, (c) upregulation by genotoxic insults like the aforementioned ultraviolet radiation exposure, or (d) lack of functional E6 expression. 34 , 70 , 76 , 100 For these reasons, the p53 protein was not included as a molecular parameter in the present meta‐analysis.
The present study has some limitations. First, the inclusion criterion for study selection that “the results of the molecular parameter in HNSCC of NSND had to be reported in the title or abstract” might have introduced selection bias, as the parameter could have been portrayed in the tables or full text without an explicit description of this criterion in the abstract. However, as the main aim of the present systematic review was to provide an overview of potential molecular parameters underlying head and neck carcinogenesis in NSND, reporting of important parameters in the title or abstract was assumed. Secondly, molecular parameters may have been found less potential in other studies and therefore may not have been published, resulting in publication bias. To limit this bias, articles reporting that HPV, p16 overexpression, TP53 mutations, and p53 protein expression play no role in the head and neck carcinogenesis of NSND were included as well. Thirdly, the methodological quality assessment of the included studies showed great heterogeneity in internal and external validity across studies. Therefore, the focus during critical appraisal was on well‐described detection methods and reproducibility of the study protocol for inclusion in the meta‐analysis. Fourthly, older studies could have reported on p16 expression without knowing its correlation to HPV infection in OPSCC, therefore not applying the nowadays accepted cutoff value of ≥70% positivity or diffuse intense/strong staining in tumor tissue. As a result, possible HPV positive cases could have been excluded from the meta‐analysis, which may have an impact on the reported HPV prevalence in this study. Finally, analyses of the data on nonsmokers and nondrinkers had to be performed separately as in the majority of the studies it was unclear if these groups showed overlap in tobacco and alcohol consumption. Moreover, studies were not excluded based on their definition of the construct NSND, so consumption of either tobacco or alcohol might have played a minor role.
This systematic review summarizes the current knowledge about the underlying carcinogenic mechanisms in NSND. HNSCC in these patients is more often related to the molecular parameters HPV and tumor protein p16 overexpression compared to tumors of SD. In a third of virus‐negative tumors, TP53 mutations were detected with a mutational profile associated with aging and ultraviolet light exposure (in lip squamous cell carcinoma) rather than to tobacco consumption. Future research should consider a strict definition of the construct nonsmoker (ie, <100 tobacco products/lifetime), whereas a less strict definition of the construct nondrinker could be opted for (ie, <1 alcoholic drink/day). For the sporadically reported molecular parameters in tumors of NSND, such as immune response and checkpoint factors including the INFγ and NFKB pathways, larger studies are needed to confirm the value of these molecular parameters in cancer diagnosis, prognosis, individualized therapy selection, or therapy effect evaluation in NSND.
Supporting information
Supplementary Table 1 Literature search
Supplementary Table 2 Study quality assessment criteria
Supplementary Table 3 Sporadically reported molecular parameters in head and neck squamous cell carcinoma of non‐smokers and non‐drinkers as presented in Figure 2
Supplementary Table 4 REMARK based quality assessment of 57 studies reporting on HPV, p16, p53, or TP53 mutations in head and neck squamous cell carcinoma of non‐smokers and non‐drinkers.
Abbreviations: REMARK: REporting recommendations for tumour MARKer prognostic studies27; HPV: human papillomavirus; NSND: non‐smokers and non‐drinkers; PCR: polymerase chain reaction; Seq: sequencing; IHC: immunohistochemistry; ISH: in situ hybridization
Supplementary Figure 1 Sensitivity analyses evaluating the statistical reliability of the data by only retaining studies with at least ten patients in both the non‐smoking/non‐drinking and smoking/drinking groups. Since exclusion of these studies did not change the conclusions, all studies remained included in the meta‐analysis (Figure 3).
ACKNOWLEDGEMENTS
We thank Bjorn Winkens, Department of Methodology and Statistics, Maastricht University, Maastricht, Netherlands, for his helpful comments regarding the sensitivity analysis.
Mulder FJ, Pierssens DDCG, Baijens LWJ, Kremer B, Speel E‐JM. Evidence for different molecular parameters in head and neck squamous cell carcinoma of nonsmokers and nondrinkers: Systematic review and meta‐analysis on HPV, p16, and TP53 . Head & Neck. 2021;43:303–322. 10.1002/hed.26513
Meetings at which the manuscript was presented: 7th World Congress of the International Academy of Oral Oncology; September 1, 2019; Rome, Italy. 235th Meeting of the Dutch Society of Otorhinolaryngology and Head & Neck Surgery, November 22, 2019; Utrecht, Netherlands.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Dal Maso L, Torelli N, Biancotto E, et al. Combined effect of tobacco smoking and alcohol drinking in the risk of head and neck cancers: a re‐analysis of case‐control studies using bi‐dimensional spline models. Eur J Epidemiol. 2016;31:385‐393. [DOI] [PubMed] [Google Scholar]
- 2. Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709‐720. [DOI] [PubMed] [Google Scholar]
- 3. Leemans CR, Snijders PJF, Brakenhoff RH. The molecular landscape of head and neck cancer. Nat Rev Cancer. 2018;18:269‐282. [DOI] [PubMed] [Google Scholar]
- 4. Habbous S, Chu KP, Lau H, et al. Human papillomavirus in oropharyngeal cancer in Canada: analysis of 5 comprehensive cancer centres using multiple imputation. CMAI. 2017;189:E1030‐e1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dahlstrom KR, Little JA, Zafereo ME, Lung M, Wei Q, Sturgis EM. Squamous cell carcinoma of the head and neck in never smoker‐never drinkers: a descriptive epidemiologic study. Head Neck. 2008;30:75‐84. [DOI] [PubMed] [Google Scholar]
- 6. Durr ML, Li D, Wang SJ. Oral cavity squamous cell carcinoma in never smokers: analysis of clinicopathologic characteristics and survival. Am J Otolaryngol. 2013;34:388‐393. [DOI] [PubMed] [Google Scholar]
- 7. Farshadpour F, Hordijk G, Koole R, Slootweg P. Non‐smoking and non‐drinking patients with head and neck squamous cell carcinoma: a distinct population. Oral Diseases. 2007;13:239‐243. [DOI] [PubMed] [Google Scholar]
- 8. Harris SL, Kimple RJ, Hayes DN, Couch ME, Rosenman JG. Never‐smokers, never‐drinkers: unique clinical subgroup of young patients with head and neck squamous cell cancers. Head Neck. 2010;32:499‐503. [DOI] [PubMed] [Google Scholar]
- 9. Koo K, Barrowman R, McCullough M, Iseli T, Wiesenfeld D. Non‐smoking non‐drinking elderly females: a clinically distinct subgroup of oral squamous cell carcinoma patients. International Journal of Oral and Maxillofacial Surgery. 2013;42:929‐933. [DOI] [PubMed] [Google Scholar]
- 10. Kruse AL, Bredell M, Grätz KW. Oral squamous cell carcinoma in non‐smoking and non‐drinking patients. Head Neck Oncol. 2010;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wiseman SM, Swede H, Stoler DL, et al. Squamous cell carcinoma of the head and neck in nonsmokers and nondrinkers: an analysis of clinicopathologic characteristics and treatment outcomes. Ann Surg Oncol. 2003;10:551‐557. [DOI] [PubMed] [Google Scholar]
- 12. O'Rorke MA, Ellison MV, Murray LJ, Moran M, James J, Anderson LA. Human papillomavirus related head and neck cancer survival: a systematic review and meta‐analysis. Oral Oncol. 2012;48:1191‐1201. [DOI] [PubMed] [Google Scholar]
- 13. Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta‐analysis. Int J Cancer. 2007;121:1813‐1820. [DOI] [PubMed] [Google Scholar]
- 14. Golusinski W, Leemans R, Dietz A. HPV Infection in Head and Neck Cancer. Vol 206 Switserland: Springer; 2017. [Google Scholar]
- 15. O'Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV‐related oropharyngeal cancer by the international collaboration on 0ropharyngeal cancer network for staging (ICON‐S): a multicentre cohort study. Lancet Oncol. 2016;17:440‐451. [DOI] [PubMed] [Google Scholar]
- 16. Brierley JDG, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours 8th Edition. New Jersey: Wiley‐Blackwell; 2016. [Google Scholar]
- 17. Andrews E, Seaman WT, Webster‐Cyriaque J. Oropharyngeal carcinoma in non‐smokers and non‐drinkers: a role for HPV. Oral Oncol. 2009;45:486‐491. [DOI] [PubMed] [Google Scholar]
- 18. Farshadpour F, Konings S, Speel EJ, et al. Human papillomavirus and Oropharyngeal squamous cell carcinoma: a case‐control study regarding tobacco and alcohol consumption. Patholog Res Int. 2011;2011:806345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laco J, Vosmikova H, Novakova V, et al. The role of high‐risk human papillomavirus infection in oral and oropharyngeal squamous cell carcinoma in non‐smoking and non‐drinking patients: a clinicopathological and molecular study of 46 cases. Virchows Archiv. 2011;458:179‐187. [DOI] [PubMed] [Google Scholar]
- 20. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous‐cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856‐1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE‐012): an open‐label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956‐965. [DOI] [PubMed] [Google Scholar]
- 23. Ferris RL. Immunology and immunotherapy of head and neck Cancer. J Clin Oncol. 2015;33:3293‐3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Almangush A, Heikkinen I, Makitie AA, et al. Prognostic biomarkers for oral tongue squamous cell carcinoma: a systematic review and meta‐analysis. Br J Cancer. 2017;117:856‐866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rivera C, Oliveira AK, Costa RAP, De Rossi T, Paes Leme AF. Prognostic biomarkers in oral squamous cell carcinoma: a systematic review. Oral Oncol. 2017;72:38‐47. [DOI] [PubMed] [Google Scholar]
- 27. McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005;93:387‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Field JK, Spandidos DA, Malliri A, Gosney JR, Yiagnisis M, Stell PM. Elevated P53 expression correlates with a history of heavy smoking in squamous cell carcinoma of the head and neck. Br J Cancer. 1991;64:573‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsimplaki E, Argyri E, Xesfyngi D, Daskalopoulou D, Stravopodis DJ, Panotopoulou E. Prevalence and expression of human papillomavirus in 53 patients with oral tongue squamous cell carcinoma. Anticancer Res. 2014;34:1021‐1025. [PubMed] [Google Scholar]
- 31. Oliveira LR, Ribeiro‐Silva A, Zambelli Ramalho LN, Simoes AL, Zucoloto S. HPV infection in Brazilian oral squamous cell carcinoma patients and its correlation with clinicopathological outcomes. Mol Med Rep. 2008;1:123‐129. [PubMed] [Google Scholar]
- 32. Amsbaugh MJ, Yusuf M, Cash E, et al. Distribution of cervical lymph node metastases from squamous cell carcinoma of the oropharynx in the era of risk stratification using human papillomavirus and smoking status. Int J Radiat Oncol Biol Phys. 2016;96:349‐353. [DOI] [PubMed] [Google Scholar]
- 33. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Angiero F, Gatta LB, Seramondi R, et al. Frequency and role of HPV in the progression of epithelial dysplasia to oral cancer. Anticancer Res. 2010;30:3435‐3440. [PubMed] [Google Scholar]
- 35. Antonsson A, Neale RE, Boros S, et al. Human papillomavirus status and p16(INK4A) expression in patients with mucosal squamous cell carcinoma of the head and neck in Queensland. Australia. Cancer Epidemiol. 2015;39:174‐181. [DOI] [PubMed] [Google Scholar]
- 36. Bragelmann J, Dagogo‐Jack I, El Dinali M, et al. Oral cavity tumors in younger patients show a poor prognosis and do not contain viral RNA. Oral Oncol. 2013;49:525‐533. [DOI] [PubMed] [Google Scholar]
- 37. Chen F, Yan L, Liu F, et al. Oral human papillomavirus infection, sexual behaviors and risk of oral squamous cell carcinoma in southeast of China: a case‐control study. J Clin Virol. 2016;85:7‐12. [DOI] [PubMed] [Google Scholar]
- 38. Chen WC, Chuang HC, Lin YT, Huang CC, Chien CY. Clinical impact of human papillomavirus in laryngeal squamous cell carcinoma: a retrospective study. PeerJ. 2017;5:e3395 10.7717/peerj.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen X, Gao L, Sturgis EM, et al. HPV16 DNA and integration in normal and malignant epithelium: implications for the etiology of laryngeal squamous cell carcinoma. Ann Oncol. 2017;28:1105‐1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chuang CY, Sung WW, Wang L, et al. Differential impact of IL‐10 expression on survival and relapse between HPV16‐positive and ‐negative oral squamous cell carcinomas. PLoS One. 2012;7:e47541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dediol E, Sabol I, Virag M, Grce M, Muller D, Manojlović S. HPV prevalence and p16 INK a overexpression in non‐smoking non‐drinking oral cavity cancer patients. Oral Diseases. 2016;22:517‐522. [DOI] [PubMed] [Google Scholar]
- 42. Descamps G, Karaca Y, Lechien JR, et al. Classical risk factors, but not HPV status, predict survival after chemoradiotherapy in advanced head and neck cancer patients. J Cancer Res Clin Oncol. 2016;142:2185‐2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Farnebo L, Stjernstrom A, Fredrikson M, Ansell A, Garvin S, Thunell LK. DNA repair genes XPC, XPD, XRCC1, and XRCC3 are associated with risk and survival of squamous cell carcinoma of the head and neck. DNA Repair (Amst). 2015;31:64‐72. [DOI] [PubMed] [Google Scholar]
- 44. Fouret P, Monceaux G, Temam S, Lacourreye L, St Guily JL. Human papillomavirus in head and neck squamous cell carcinomas in nonsmokers. Arch Otolaryngol Head Neck Surg. 1997;123:513‐516. [DOI] [PubMed] [Google Scholar]
- 45. Gillison ML, D'Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16‐positive and human papillomavirus type 16‐negative head and neck cancers. J Natl Cancer Inst. 2008;100:407‐420. [DOI] [PubMed] [Google Scholar]
- 46. Gonzalez‐Ramirez I, Irigoyen‐Camacho ME, Ramirez‐Amador V, et al. Association between age and high‐risk human papilloma virus in Mexican oral cancer patients. Oral Dis. 2013;19:796‐804. [DOI] [PubMed] [Google Scholar]
- 47. Hafkamp HC, Manni JJ, Haesevoets A, et al. Marked differences in survival rate between smokers and nonsmokers with HPV 16‐associated tonsillar carcinomas. Int J Cancer. 2008;122:2656‐2664. [DOI] [PubMed] [Google Scholar]
- 48. Hoffmann M, Quabius ES, Tribius S, et al. Influence of HPV‐status on survival of patients with tonsillar carcinomas (TSCC) treated by CO2−laser surgery plus risk adapted therapy—a 10 year retrospective single Centre study. Cancer Lett. 2018;413:59‐68. [DOI] [PubMed] [Google Scholar]
- 49. Hong A, Lee CS, Jones D, et al. Rising prevalence of human papillomavirus‐related oropharyngeal cancer in Australia over the last 2 decades. Head Neck. 2016;38:743‐750. [DOI] [PubMed] [Google Scholar]
- 50. Joo YH, Lee YS, Cho KJ, et al. Characteristics and prognostic implications of high‐risk HPV‐associated hypopharyngeal cancers. PLoS One. 2013;8(11):e78718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li R, Faden DL, Fakhry C, et al. Clinical, genomic, and metagenomic characterization of oral tongue squamous cell carcinoma in patients who do not smoke. Head Neck. 2015;37:1642‐1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maruyama H, Yasui T, Ishikawa‐Fujiwara T, et al. Human papillomavirus and p53 mutations in head and neck squamous cell carcinoma among Japanese population. Cancer Sci. 2014;105:409‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mena M, Taberna M, Tous S, et al. Double positivity for HPV‐DNA/p16ink4a is the biomarker with strongest diagnostic accuracy and prognostic value for human papillomavirus related oropharyngeal cancer patients. Oral Oncol. 2018;78:137‐144. [DOI] [PubMed] [Google Scholar]
- 54. Peterson LA, Bellile EL, Wolf GT, et al. Cigarette use, comorbidities, and prognosis in a prospective head and neck squamous cell carcinoma population. Head Neck. 2016;38:1810‐1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Platek AJ, Jayaprakash V, Merzianu M, et al. Smoking cessation is associated with improved survival in oropharynx cancer treated by chemoradiation. Laryngoscope. 2016;126:2733‐2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Poling JS, Ma XJ, Bui S, et al. Human papillomavirus (HPV) status of non‐tobacco related squamous cell carcinomas of the lateral tongue. Oral Oncol. 2014;50:306‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Quabius ES, Gorogh T, Fischer GS, et al. The antileukoprotease secretory leukocyte protease inhibitor (SLPI) and its role in the prevention of HPV‐infections in head and neck squamous cell carcinoma. Cancer Lett. 2015;357:339‐345. [DOI] [PubMed] [Google Scholar]
- 58. Schlecht NF, Burk RD, Adrien L, et al. Gene expression profiles in HPV‐infected head and neck cancer. J Pathol. 2007;213:283‐293. [DOI] [PubMed] [Google Scholar]
- 59. Siebers TJH, Merkx MAW, Slootweg PJ, Melchers WJG, van Cleef P, Wilde PCM. No high‐risk HPV detected in SCC of the oral tongue in the absolute absence of tobacco and alcohol—a case study of seven patients. Oral Maxillofacial Surg. 2008;12:185‐188. [DOI] [PubMed] [Google Scholar]
- 60. Simonato LE, Garcia JF, Sundefeld MLMM, Mattar NJ, Veronese LA, Miyahara GI. Detection of HPV in mouth floor squamous cell carcinoma and its correlation with clinicopathologic variables, risk factors and survival. J Oral Pathol Med. 2008;37:593‐598. [DOI] [PubMed] [Google Scholar]
- 61. Tachezy R, Klozar J, Salakova M, et al. HPV and other risk factors of oral cavity/oropharyngeal cancer in The Czech Republic. Oral Dis. 2005;11:181‐185. [DOI] [PubMed] [Google Scholar]
- 62. Vatca M, Lucas JT, Laudadio J, et al. Retrospective analysis of the impact of HPV status and smoking on mucositis in patients with oropharyngeal squamous cell carcinoma treated with concurrent chemotherapy and radiotherapy. Oral Oncol. 2014;50:869‐876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wangsa D, Chowdhury SA, Ryott M, et al. Phylogenetic analysis of multiple FISH markers in oral tongue squamous cell carcinoma suggests that a diverse distribution of copy number changes is associated with poor prognosis. Int J Cancer. 2016;138:98‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xu Y, Liu S, Yi H, et al. Human papillomavirus infection in 674 Chinese patients with laryngeal squamous cell carcinoma. PLoS One. 2014;9:e115914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16‐positive and p16‐negative oropharyngeal cancer. J Clin Oncol. 2012;30:2102‐2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Haas S, Hormann K, Bosch FX. Expression of cell cycle proteins in head and neck cancer correlates with tumor site rather than tobacco use. Oral Oncol. 2002;38:618‐623. [DOI] [PubMed] [Google Scholar]
- 67. Heaton CM, Durr ML, Tetsu O, Van Zante A, Wang SJ. TP53 and CDKN2a mutations in never‐smoker oral tongue squamous cell carcinoma. Laryngoscope. 2014;124:E267‐E273. [DOI] [PubMed] [Google Scholar]
- 68. Hess CB, Rash DL, Daly ME, et al. Competing causes of death and medical comorbidities among patients with human papillomavirus‐positive vs human papillomavirus‐negative oropharyngeal carcinoma and impact on adherence to radiotherapy. JAMA Otolaryngol Head Neck Surg. 2014;140:312‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kalfert D, Celakovsky P, Laco J, Ludvikova M. The role of protein p16(INK4a) in glottic laryngeal squamous cell carcinoma. Pathol Oncol Res. 2014;20:909‐915. [DOI] [PubMed] [Google Scholar]
- 70. Karpathiou G, Monaya A, Forest F, et al. p16 and p53 expression status in head and neck squamous cell carcinoma: a correlation with histological, histoprognostic and clinical parameters. Pathology. 2016;48:341‐348. [DOI] [PubMed] [Google Scholar]
- 71. Mafune A, Hama T, Suda T, et al. Homozygous deletions of UGT2B17 modifies effects of smoking on TP53‐mutations and relapse of head and neck carcinoma. BMC Cancer. 2015;15:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ralli M, Singh S, Yadav SP, Sharma N, Verma R, Sen R. Assessment and clinicopathological correlation of p16 expression in head and neck squamous cell carcinoma. J Cancer Res Ther. 2016;12:232‐237. [DOI] [PubMed] [Google Scholar]
- 73. Silva SD, Nonogaki S, Soares FA, Kowalski LP. P16 (INK4a) has clinicopathological and prognostic impact on oropharynx and larynx squamous cell carcinoma. Braz J Med Biol Res. 2012;45:1327‐1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ye A, Bradley KL, Kader H, Wu J, Hay JH. Patterns of relapse in squamous cell carcinoma of the tonsil—unilateral vs bilateral radiation in the HPV‐era. Cureus. 2015;7:e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhao D, Wang S‐H, Feng Y, Hua C‐G, Zhao J, Tang X‐F. Intratumoral c‐Met expression is associated with vascular endothelial growth factor C expression, lymphangiogenesis, and lymph node metastasis in oral squamous cell carcinoma: implications for use as a prognostic marker. Human Pathol. 2011;42:1514‐1523. [DOI] [PubMed] [Google Scholar]
- 76. Fernandez‐Acenero MJ, Larach F, Aramendi T, Ortega P. Possible prognostic role of p53 expression in laryngeal squamous cell carcinoma of non‐smokers, non‐alcoholic patients. Acta Oto‐Laryngologica. 2008;128:1385‐1388. [DOI] [PubMed] [Google Scholar]
- 77. Matthews JB, Scully C, Jovanovic A, Van der Waal I, Yeudall WA, Prime SS. Relationship of tobacco/alcohol use to p53 expression in patients with lingual squamous cell carcinomas. Eur J Cancer Part B Oral Oncol. 1993;29:285‐289. [DOI] [PubMed] [Google Scholar]
- 78. Faden DL, Arron ST, Heaton CM, DeRisi J, South AP, Wang SJ. Targeted next‐generation sequencing of TP53 in oral tongue carcinoma from non‐smokers. J Otolaryngol Head Neck Surg. 2016;45:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hong A, Zhang X, Jones D, et al. Relationships between p53 mutation, HPV status and outcome in oropharyngeal squamous cell carcinoma. Radiother Oncol. 2016;118:342‐349. [DOI] [PubMed] [Google Scholar]
- 80. Mirghani H, Lacroix L, Rossoni C, et al. Does smoking alter the mutation profile of human papillomavirus‐driven head and neck cancers? Eur J Cancer. 2018;94:61‐69. [DOI] [PubMed] [Google Scholar]
- 81. Ostwald C, Gogacz P, Hillmann T, et al. p53 mutational spectra are different between squamous‐cell carcinomas of the lip and the oral cavity. Int J Cancer. 2000;88:82‐86. [DOI] [PubMed] [Google Scholar]
- 82. Pickering CR, Zhang J, Neskey DM, et al. Squamous cell carcinoma of the oral tongue in young non‐smokers is genomically similar to tumors in older smokers. Clin Cancer Res. 2014;20:3842‐3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tan DS, Wang W, Leong HS, et al. Tongue carcinoma infrequently harbor common actionable genetic alterations. BMC Cancer. 2014;14:679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zanaruddin SN, Yee PS, Hor SY, et al. Common oncogenic mutations are infrequent in oral squamous cell carcinoma of Asian origin. PLoS One. 2013;8:e80229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Farshadpour F, Roepman P, Hordijk GJ, Koole R, Slootweg PJ. A gene expression profile for non‐smoking and non‐drinking patients with head and neck cancer. Oral Diseases. 2012;18:178‐183. [DOI] [PubMed] [Google Scholar]
- 86. Foy JP, Bertolus C, Michallet MC, et al. The immune microenvironment of HPV‐negative oral squamous cell carcinoma from never‐smokers and never‐drinkers patients suggests higher clinical benefit of IDO1 and PD1/PD‐L1 blockade. Ann Oncol. 2017;28:1934‐1941. [DOI] [PubMed] [Google Scholar]
- 87. Soares PDO, Cury PM, Lopez RVM, et al. GTSP1 expression in non‐smoker and nondrinker patients with squamous cell carcinoma of the head and neck. PLoS One. 2017;12(8):e0182600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Alexandrov LB, Ju YS, Haase K, et al. Mutational signatures associated with tobacco smoking in human cancer. Science. 2016;354:618‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hashibe M, Brennan P, Benhamou S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99:777‐789. [DOI] [PubMed] [Google Scholar]
- 90. Berthiller J, Straif K, Agudo A, et al. Low frequency of cigarette smoking and the risk of head and neck cancer in the INHANCE consortium pooled analysis. Int J Epidemiol. 2016;45:835‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467‐475. [DOI] [PubMed] [Google Scholar]
- 92. Castellsague X, Alemany L, Quer M, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108:djv403. [DOI] [PubMed] [Google Scholar]
- 93. Mehanna H, Robinson M, Hartley A, et al. Radiotherapy plus cisplatin or cetuximab in low‐risk human papillomavirus‐positive oropharyngeal cancer (De‐ESCALaTE HPV): an open‐label randomised controlled phase 3 trial. Lancet. 2019;393:51‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mirghani H, Blanchard P. Treatment de‐escalation for HPV‐driven oropharyngeal cancer: where do we stand? Clin Transl Radiat Oncol. 2018;8:4‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Smeets SJ, Hesselink AT, Speel EJ, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007;121:2465‐2472. [DOI] [PubMed] [Google Scholar]
- 96. Jung AC, Briolat J, Millon R, et al. Biological and clinical relevance of transcriptionally active human papillomavirus (HPV) infection in oropharynx squamous cell carcinoma. Int J Cancer. 2010;126:1882‐1894. [DOI] [PubMed] [Google Scholar]
- 97. Alexandrov LB, Nik‐Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Poeta ML, Manola J, Goldwasser MA, et al. TP53 mutations and survival in squamous‐cell carcinoma of the head and neck. N Engl J Med. 2007;357:2552‐2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Skinner HD, Sandulache VC, Ow TJ, et al. TP53 disruptive mutations lead to head and neck cancer treatment failure through inhibition of radiation‐induced senescence. Clin Cancer Res. 2012;18:290‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hafkamp HC, Speel EJ, Haesevoets A, et al. A subset of head and neck squamous cell carcinomas exhibits integration of HPV 16/18 DNA and overexpression of p16INK4A and p53 in the absence of mutations in p53 exons 5‐8. Int J Cancer. 2003;107:394‐400. [DOI] [PubMed] [Google Scholar]
- 101. Taylor D, Koch WM, Zahurak M, Shah K, Sidransky D, Westra WH. Immunohistochemical detection of p53 protein accumulation in head and neck cancer: correlation with p53 gene alterations. Hum Pathol. 1999;30:1221‐1225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 Literature search
Supplementary Table 2 Study quality assessment criteria
Supplementary Table 3 Sporadically reported molecular parameters in head and neck squamous cell carcinoma of non‐smokers and non‐drinkers as presented in Figure 2
Supplementary Table 4 REMARK based quality assessment of 57 studies reporting on HPV, p16, p53, or TP53 mutations in head and neck squamous cell carcinoma of non‐smokers and non‐drinkers.
Abbreviations: REMARK: REporting recommendations for tumour MARKer prognostic studies27; HPV: human papillomavirus; NSND: non‐smokers and non‐drinkers; PCR: polymerase chain reaction; Seq: sequencing; IHC: immunohistochemistry; ISH: in situ hybridization
Supplementary Figure 1 Sensitivity analyses evaluating the statistical reliability of the data by only retaining studies with at least ten patients in both the non‐smoking/non‐drinking and smoking/drinking groups. Since exclusion of these studies did not change the conclusions, all studies remained included in the meta‐analysis (Figure 3).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


