Figure 2.
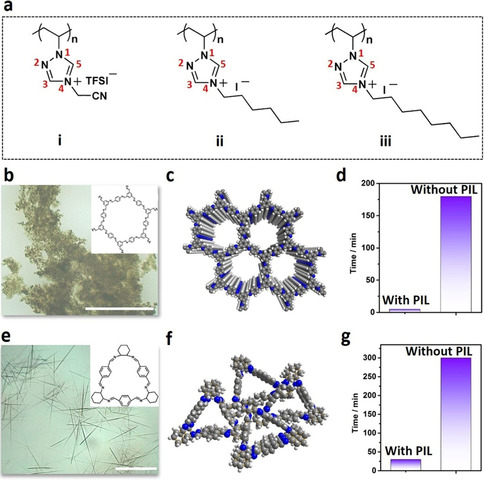
a) Various PILs used for accelerating the crystallization of open organics. The positions of atoms in the triazolium ring are numerically labeled. i) Poly(4‐cyanomethyl‐1‐vinyl‐1,2,4‐triazolium bis(trifluoromethane sulfonyl)imide), ii) poly(4‐hexyl‐1‐vinyl‐1,2,4‐triazolium iodide) (Ptriaz), iii) poly(4‐octyl‐1‐vinyl‐1,2,4‐triazolium iodide). The microscope image of crystals (crystallite for COFs, the TEM images are shown in the Supporting Information) and corresponding chemical structures growth with different PILs additive, b),c) BTA‐PED COF with poly(4‐cyanomethyl‐1‐vinyl‐1,2,4‐triazolium bis(trifluoromethane sulfonyl)imide); e),f) TPA‐DACH macrocycle with poly(4‐octyl‐1‐vinyl‐1,2,4‐triazolium iodide), scale bar: 100 μm. d),g) The comparison of crystallizing time for growth of similar sized crystallites (ca. 36 nm for BTA‐PED COF; Supporting Information, Figure S3) and crystals (ca. 80 μm for TPA‐DACH macrocycle; Supporting Information, Figure S4) with and without PILs.
