Figure 5.
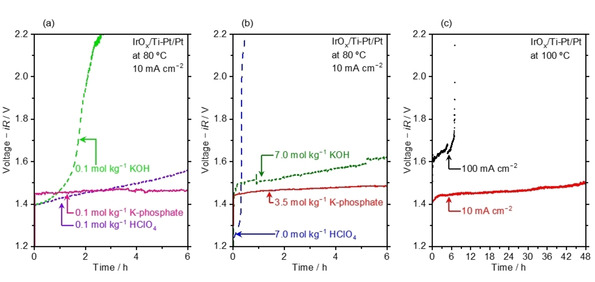
Water electrolysis performance in concentrated buffer solutions. (a) Chronopotentiometry (CP) profile performed at 10 mA cm−2 and 80 °C in electrolyte solutions of 0.1 mol kg−1 KOH, 0.1 mol kg−1 HClO4, and 0.1 mol kg−1 K‐phosphate. (b) CP profile performed at 10 mA cm−2 and 80 °C in electrolyte solutions of 7.0 mol kg−1 KOH, 7.0 mol kg−1 HClO4, and 3.5 mol kg−1 K‐phosphate. These profiles are the average of the experiments for 3.5 mol kg−1 K‐phosphate, 7.0 mol kg−1 HClO4, and KOH (see Figure S10 for raw data. (c) Overall water electrolysis performance was accessed by CP at 10 and 100 mA cm−2 in 4.1 mol kg−1 K‐phosphate solutions at 100 °C. All measurements were performed in the two‐electrode configuration using IrOx/Ti mesh and Pt/Pt mesh as anode and cathode with a geometric surface area of 1.0 cm2, respectively, under Ar bubbling. The voltage displayed in the figure has been iR‐corrected with measured impedance value unless otherwise noted. The pH level of K‐phosphate solutions was adjusted to 7.0 at 25 °C prior to the measurements.
