Summary
Diabetes is a major health issue of increasing prevalence. ß‐cell replacement, by pancreas or islet transplantation, is the only long‐term curative option for patients with insulin‐dependent diabetes. Despite good functional results, pancreas transplantation remains a major surgery with potentially severe complications. Islet transplantation is a minimally invasive alternative that can widen the indications in view of its lower morbidity. However, the islet isolation procedure disrupts their vasculature and connection to the surrounding extracellular matrix, exposing them to ischemia and anoikis. Implanted islets are also the target of innate and adaptive immune attacks, thus preventing robust engraftment and prolonged full function. Generation of organoids, defined as functional 3D structures assembled with cell types from different sources, is a strategy increasingly used in regenerative medicine for tissue replacement or repair, in a variety of inflammatory or degenerative disorders. Applied to ß‐cell replacement, it offers the possibility to control the size and composition of islet‐like structures (pseudo‐islets), and to include cells with anti‐inflammatory or immunomodulatory properties. In this review, we will present approaches to generate islet cell organoids and discuss how these strategies can be applied to the generation of a bioartificial pancreas for the treatment of type 1 diabetes.
Keywords: bioengineering, cell transplantation, islet transplantation, organoids, type 1 diabetes
Introduction
Diabetes represents a major health issue with a current prevalence of 463 million affected adults worldwide and an expected prevalence of 578 million in 2030 [1]. In the long term, poor glycemic control puts diabetic patients at risk of developing micro‐ and macro‐vascular complications leading to cardiopathy, neuropathy, retinopathy, and nephropathy [2]. Type 1 diabetes (T1D) is characterized by autoimmune destruction of insulin‐producing ß cells and the current basis of treatment is by exogenous insulin administration. Despite recent improvements, insulin injection only imperfectly controls blood sugar levels, which may lead to two major clinical issues: the development of chronic complications, including end‐stage renal failure and the need of kidney transplantation, and life‐threatening problematic hypoglycemia. Beta‐cell replacement by transplantation of the whole pancreas or isolated islets of Langerhans is an efficient way of restoring euglycemia, thus preventing the occurrence of severe hypoglycemia and protecting kidney grafts from the recurrence of diabetic nephropathy [3]. Pancreas transplantation, usually performed simultaneously with a kidney (SPK) in diabetic patients with end‐stage renal insufficiency, is a major surgical procedure associated with significant morbidity [4]. Islet transplantation (IT) is a minimally invasive procedure, showing promising functional results that can be offered to a wider range of patients with T1D. However, the isolated islets have to face several challenges. The isolation procedure and engraftment process lead to a significant loss of insulin‐producing tissue, due to isolation‐related damage, loss of vascularization, loss of extracellular matrix, and an inflammatory microenvironment at the site of implantation [5]. These phenomena lead to the need for multiple donors in order to achieve insulin independence as well as to attrition of islet graft function over time [5] (Fig. 1). In the past decades, remarkable progress has been achieved in the enhancement of islet survival and engraftment, such as refinements in the islet isolation procedure, the design of steroid‐free immunosuppressive regimens, and the development of anti‐inflammatory strategies [6]. In the research field, further advances have shown promise in addressing the issues of immune protection and shortage of insulin‐producing tissue. One strategy that has been widely studied, especially in tissue engineering, is the generation of organoids allowing to recreate organs from embryonic and/or adult stem cells [7]. This technology has been especially attractive in pancreatic islet research because of its ability to control the size and composition of the generated units, and the possibility to add supporting cells, such as endothelial or anti‐inflammatory cells. The aim of this review is to highlight different approaches to improve the function and maturation of insulin‐secreting organoids and discuss the perspectives and challenges of their clinical application.
Figure 1.
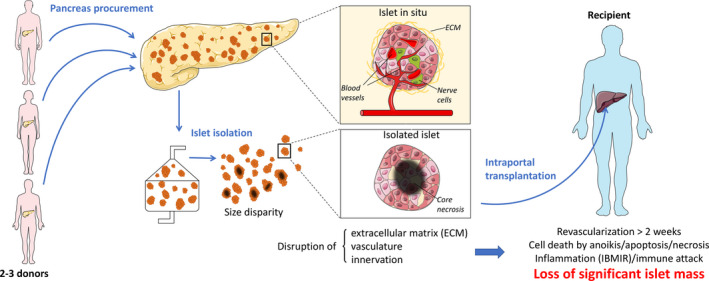
Limitations of clinical islet transplantation. The isolation process is responsible for the loss and disruption of the ECM, vasculature, and innervation of the islets. In addition to the inflammatory and immune attacks, this process results in the loss of an important proportion of the islet mass. IBMIR: instant blood mediated inflammatory reaction.
The islet of Langerhans: a connected object
Islets of Langerhans are endocrine cell aggregates, representing less than 5% of the total pancreas volume and with a mean diameter of 100–150 μm [4, 8, 9]. In humans, an islet equivalent (IEQ, defined as a standardized islet with a 150 μm diameter) contains approximately 1500 cells [10] and is composed of 60% insulin‐secreting cells (ß cells) and 30% glucagon‐secreting cells (α cells) [11, 12]. The remaining 10% is composed of somatostatin‐secreting cells (δ cells), pancreatic polypeptide‐secreting cells (γ or PP cells), and ghrelin‐secreting cells (ε cells) [11, 12]. In addition to endocrine cells, islets contain stromal cells, macrophages, neuronal elements, endothelial cells (EC), and pericytes, altogether representing <5% [10]. This indicates that more than a simple cell aggregate, the islet is a functional mini‐organ with its own innervation [13] and complex intercellular communications [14]. In order to exert their endocrine functions, islet cells have to receive and process signals coming from the bloodstream and/or interstitial space such as nutrients, hormones, and neurotransmitters but also inputs from their innervation. Cell‐to‐cell contacts are therefore crucial for hormone release. In addition to autocrine, paracrine, and endocrine pathways, cells communicate via intercellular connections using cell adhesion molecules (cadherins), gap junctions, and ephrin receptors and ligands [15, 16]. Cell adhesion molecules are important in the development of islet architecture and function. For example, lack of neural cell adhesion molecule (N‐CAM) impairs islet cell organization and insulin secretion [17] and cadherin‐mediated adhesion of ß cells promotes their function [18]. Signals transmitted by E‐cadherin play an important role in islet development, ß‐cell aggregation, viability, and function [18, 19, 20]. Gap junctions between ß cells allow to share small metabolites and cytoplasmic ions, such as calcium, which is essential for synchronized insulin release in response to glucose stimulation [21].
In addition to cell‐to‐cell contacts, islet cell connections with their environment are also of great importance. Islets are well‐vascularized mini‐organs, receiving 10% to 15% of the total pancreatic blood flow, with a vessel density five times greater than the exocrine part of the gland [22]. Endocrine cells are in close contact with a highly developed fenestrated capillary network allowing rapid responses to achieve optimal control of blood glucose levels. Endothelial and islet cell communications have mutual effects. Secretion of vascular endothelial growth factor (VEGF‐A) and angiopoietin‐1 (Ang‐1) by islet cells promotes the development of a functional fenestrated capillary network [23]. On the other hand, release of growth factors, such as hepatocyte growth factor (HGF), by ECs, stimulates insulin biosynthesis and secretion [24]. In addition to their essential role in angiogenesis, intra‐islet ECs synthetize ECM components, necessary for ß‐cell proliferation, differentiation, function, and survival [25, 26]. Islets are separated from the exocrine part of the pancreas by a peripheral capsule composed of fibroblasts and collagen fibers, entrapped between two basement membranes (BM) located beneath the exocrine and endocrine epithelium (peri‐islet) [15]. The peri‐islet invaginates into islets along vascular channels to form a perivascular BM. Major components of the intra‐islet perivascular BM are laminins, collagen IV, and fibronectin [25]. The importance of ß cell‐ECM interaction has been intensively studied. The lack of vascular BM significantly impairs ß‐cell proliferation and insulin gene expression. Collagen IV binding to its receptor, the α1ß1 integrin, on ß cells not only augments insulin secretion [27], but also contributes to ß‐cell differentiation and survival [28]. Signals transmitted through the α6ß1 integrin also play a major role in the regulation of ß‐cell survival [29]. Laminin‐332 is expressed in human islets, and its interaction with the integrin ß1 sub‐unit was shown to be essential for normal ß‐cell function in vitro [30, 31]. In addition, the vascular BM modulates cell behavior by acting as a source of growth factors and by trapping cytokines and others soluble signal molecules, necessary for maintaining ß‐cell phenotype and proliferation [32].
The peri‐islet BM is mainly composed of laminin and collagen IV and, to a lesser extent, of fibronectin, collagen I, III, V, and VI [33, 34]. Apart from functional support, the peri‐islet BM is essential for regulation of ß‐cell survival as suggested by the improved viability and in vitro function of incompletely isolated “mantled islets” [35, 36]. Of note, the isolation process not only disconnects islets from their peripheral BM, but also disrupts the intra‐islet BM by the loss of intra‐islet EC after isolation [37, 38]. Altogether, isolated islets are subjected to anoikis, an integrin‐mediated death signal resulting from the disruption of interaction between integrins and ECM proteins. This phenomenon is responsible for significant islet cell death in culture [39].
Organoids: building blocks for bioartificial organ construction
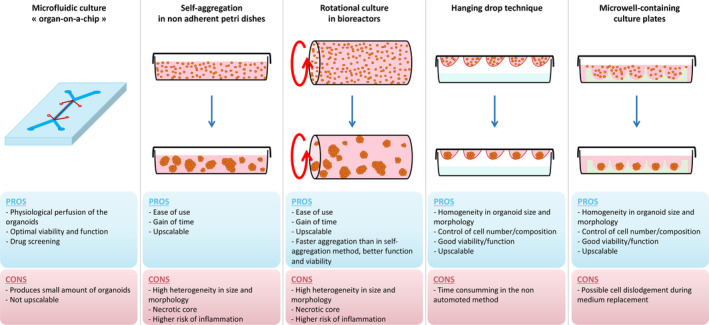
Organoids are defined as 3D cell aggregates designed with the aim to reproduce in vitro the morphology and intrinsic function of organs in vivo. Organogenesis occurs as a result of programmed cell‐to‐cell contacts and close intercellular communications [40]. In order to mimic this physiological condition, organoids have been initially generated from human embryonic stem cells (hESC) or adult mesenchymal stem cells (MSCs) and used as building blocks for tissue engineering and assembly into bioartificial organs. Numerous different methods have been developed to generate functional organoids, applying principles of cell self‐assembly [41]. Most of these approaches can be separated into microfluidic and nonmicrofluidic techniques. The microfluidic “organ‐on‐a‐chip” method is defined by the application of a continuous, pressure‐controlled, perfusion to the cells and has demonstrated good results in terms of cell aggregation and viability [42]. However, while this approach represents a valuable system for high‐throughput in vitro analyses, it is not designed for scaling up. Nonmicrofluidic methods include the hanging drop technique [43], cell self‐aggregation technique [44], and the use of microwell culture plates [45]. These methods can be adapted for large‐scale production of organoids, like for example, the automated hanging drop method [46]. The different techniques of organoid generation are summarized in Fig. 2.
Figure 2.
The different methods used for organoid generation. The upper panel of the figure describes graphically the different techniques; the lower panel describes the pros and cons of the different available methods using microfluidic or nonmicrofluidic techniques.
Over the last decade, the field of organoid science has developed considerably, notably for anti‐cancer drug development [47, 48] and in regenerative medicine [7]. The regenerative capacities of organoids can be further improved by modulating their cellular composition. Indeed, the combination of multiple cell types into organoids can better reproduce cellular interactions of complex tissues such as the liver, in which the aggregation of hepatocytes, stellate cells, and fibroblasts allows to improve viability, and function compared to monocellular cultures [49]. It was demonstrated in studies where 3D aggregates were created using adipose stem cells [50], tumor cells [51], insulin‐secreting cells [52], intestinal stem cells, and others that organoids express the hypoxia inducible factor 1‐α (HIF1‐α) in response to decreased oxygen diffusion to their core, which stimulates secretion of angiogenic and anti‐apoptotic factors. In addition, cellular 3D aggregates have shown the ability to express higher levels of stromal cell‐derived factor 1 (SDF‐1), in comparison to monolayer cultures. SDF‐1 is a hypoxia‐induced chemokine that recruits ECs for microvasculature development. Finally, combining ECs or endothelial progenitor cells with other cell types allows the development of tubular and vessel‐like structures sprouting within the organoids in vitro [53]. In addition to ECs, other supporting cells, such as MSCs, or other cells expressing anti‐inflammatory mediators can also be incorporated into the organoids [54, 55].
Pseudo‐islet: the pancreatic endocrine organoid
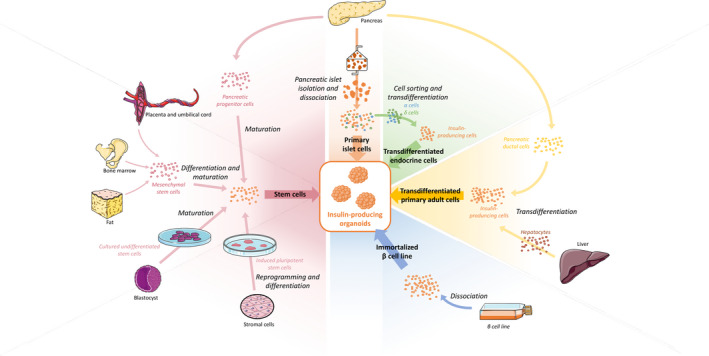
As described above, islets of Langerhans are 3D clusters composed of several cell types. Islets can be easily dissociated into single cells and reaggregated. This allows to control their size and cell composition by manipulating cell number and types. Newly generated organoids are commonly named pseudo‐islets (PIs). In addition to primary dissociated islet cells, other cell sources can be used to generate PIs, such as ß‐cell lines (e.g., MIN6 [56]), hESC [57], pancreatic stem cells [58], induced pluripotent stem cells (iPSC) [59], and other cell types using transdifferentiation such as insulin‐secreting cells derived from other endocrine cell types (alpha cells) [60] or liver cells, for instance [61] (Fig. 3).
Figure 3.
Sources of insulin‐secreting cells for organoid generation.
PIs can be generated by self‐aggregation in nonadherent petri dishes [62] or in bioreactors with rotational culture [63]. However, these techniques demonstrate a high heterogeneity in term of PIs sizes and morphology. Isolated human islets are not uniform in size, usually ranging 50–500 µm in diameter [64]. Larger islets are more prone to develop core necrosis after transplantation, until revascularization occurs [65]. Moreover, transplantation of large islets through the portal vein can elicit inflammatory reaction due to embolization of larger vessels causing liver damage. To avoid this, large‐scale generation of homogeneous, size‐controlled PIs can be achieved by using the hanging drop method or microwell culture plates. PIs have also demonstrated improved viability and function, both in vitro and in vivo, compared to native islets [66, 67], an observation attributed to the relatively small size of PIs. These findings are in line with previous reports on better in vitro performance of smaller PIs [68]. Interestingly, once transplanted, morphology and cellular arrangement of PIs changed to display a cell arrangement similar to that of native islets [69]. One step further, PIs can serve as elements for bioartificial pancreas construction, implantable in extra‐hepatic sites, thus avoiding the proinflammatory microenvironment found within the liver [70, 71, 72].
Generation of uniform PIs from dissociated single islet cells has proven to be a very effective model for gene therapy experiments, allowing, for example, homogenous lentivirus transfection of the entire PI or gene modulation using shRNA (short hairpin RNA) [73]. Finally, ß‐cell lines, usually cultivated and studied in monolayers, can be a useful cell source for PI generation and in vitro for functional studies and drug screening [74].
Validation criteria of newly formed pseudo‐islets
Generation of PIs can be considered as a novel and valuable strategy for the treatment of T1D. Therefore, it is extremely important to develop a standardized validation system. In our opinion, PIs should meet at least three important criteria:
Morphology: PIs should be small (< 150 µm diameter) and uniform in size and shape. They should also respond to the definition of spheroids in the literature: “three‐dimensional, compact, round shaped cell aggregates that do not disassemble easily and that can be easily manipulated” [42, 66, 75, 76].
Function: PIs should be able to secrete insulin in response to glucose and other secretagogues, regardless of the insulin‐secreting cell source. This can be assessed in vitro by static or perifusion secretion tests or, at the single PI level, by a reverse hemolytic plaque assay [77].
Viability: PIs must exhibit and maintain cell viability over prolonged periods of time (“a lifetime”). Viability should be assessed before implantation by standardized assays. Unfortunately, there is currently no method available to measure islet or organoid longevity.
Nontumorigenicity: PIs must demonstrate the absence of risk of uncontrolled cell proliferation, especially if gene therapy techniques or stem cell‐derived cells are used in their construction.
Improved pseudo‐islets: the benefits of adding supporting cells into organoids
As mentioned above, organoid generation offers the possibility to combine several types of cells able to provide supporting functions (Fig. 4). Several groups have used this approach, and a large variety of cell types have been assessed to this end. For instance, ECs were used to improve islet function and revascularization [78, 79]. Adding cholinergic neurons to islet cells demonstrated an increased islet function and re‐innervation in vitro [80]. Jun et al. co‐cultured islet cells with hepatocytes and, interestingly, albumin and insulin secretion were both increased in those hybrid organoids, in comparison to monocellular organoids made of hepatocyte or islet cells, respectively [81].
Figure 4.
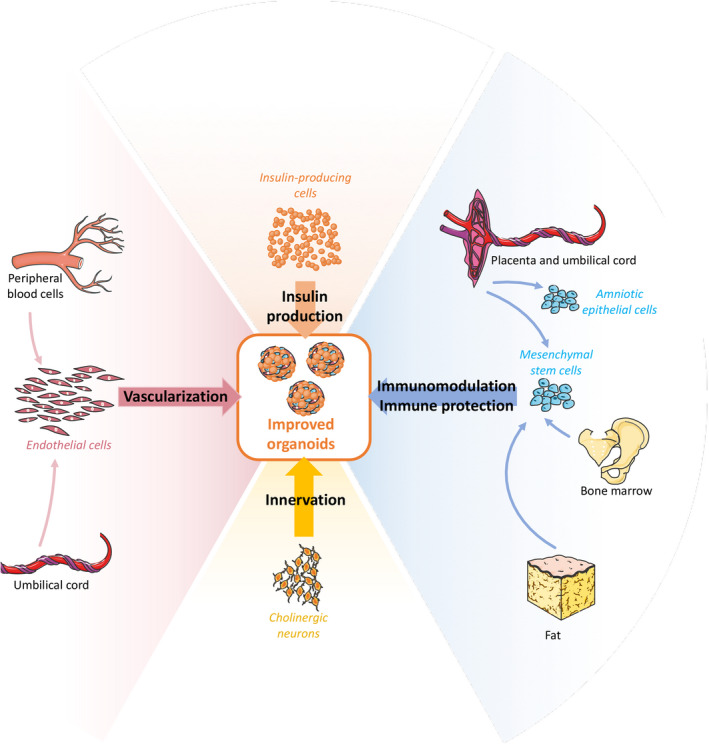
Supporting cells improving organoid function and engraftment.
The inflammatory and immunologic response against transplanted islets is detrimental for long‐term graft function. Immediately after intraportal infusion of islets, an inflammatory cascade is activated, causing the destruction of a significant proportion of the islet mass. Multicellular spheroids combining islet cells with cells expressing anti‐inflammatory and/or immunomodulatory factors could be protected from these phenomena. MSCs have been the main cell types used for this purpose [82]. Co‐culturing them with islets has enhanced revascularization, function, and engraftment thanks to their angiogenic properties [83, 84]. In addition, MSCs have differentiation capacity, which make them an interesting cell source for tissue regeneration. Over the last decades, MSCs have been used intensively, especially for inflammatory and degenerative disorders. However, MSCs harvesting is an invasive procedure, their numbers and properties decrease with donor’s age, and they have a potential for tumorigenicity [85, 86]. Amniotic epithelial cells (AECs) are an alternative source of cells with similar properties [87]. These cells are derived from the amniotic membrane and are involved in the modulation of materno‐fetal tolerance during pregnancy [88]. AECs have several advantages as a perennial source of active cells for organoid generation: They are easily accessible, inexpensive, and cause no ethical issue, since placentas are discarded after delivery; importantly, they have no tumorigenicity potential [89]. They have similar, or even more pronounced, angiogenic, antifibrotic, anti‐inflammatory and immunomodulatory than MSCs [90]. Immune‐modulatory abilities of AECs are mostly mediated by the expression and secretion of the nonclassical class I MHC antigens HLA‐G and HLA‐E that play an important part in materno‐fetal tolerance [91]. We have recently reported on the effect of combining AECs with islets or dissociated islet cells. Shielding of whole islets with AECs markedly improved their secretory function in vitro and accelerated their revascularization in vivo [92]. Similar results have been obtained in vitro and in vivo with insulin‐secreting organoids, composed of dissociated islet cells and AECs [93]. Moreover, our studies have shown that AECs have a cytoprotective effect on islet cells under hypoxic conditions, mediated by HIF‐1α. In addition to our promising results, others have demonstrated the ability of AECs to dampen the immune response against islets in both an allogenic model in vitro [94] and a xenogeneic model in vivo [95].
Taken together, these observations suggest that the inclusion of AECs inside insulin‐producing organoids has translational potential as a therapy for T1D.
Assembling organoids into a bioartificial pancreas
As developed in this review, organoids can be used as individual units, without the need to seed them onto a scaffold. They can be generated either from an allogenic or a xenogenic source, or even recipient‐derived, as described in Fig. 5. Once generated, regardless of their origin, insulin‐producing organoids can be used as building blocks, assembled and incorporated into a scaffold, to construct a bioartificial pancreas. Over the past decades, a broad variety of naturally derived and synthetic polymers, collagen gels, with or without pores, and decellularized biological matrices have been proposed for scaffold construction [96, 97, 98, 99]. While synthetic materials can be manufactured with consistent composition and can be easily fine‐tuned according to needs [97], biological scaffolds are biocompatible, and can recreate the microenvironment of the islets due to the similar composition of the ECM. The major challenge with both synthetic and biological materials is providing sufficient immune protection and adequate vascularization to the islet graft. Ideally, cell‐based tissue‐engineered constructs should be able to simultaneously allow adequate nutrient delivery to the graft, with low‐density cell loading, and be adaptable for scaling up from rodent to human dimensions [97, 100]. Fig. 6 summarizes the main classes of biomaterials used in scaffold production together with their common advantages and disadvantages.
Figure 5.
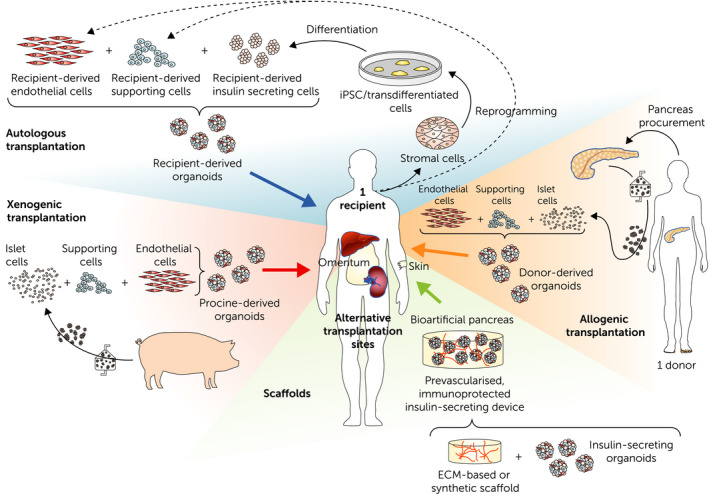
Perspectives for islet transplantation with the potential to develop either donor‐ or recipient‐derived organoids, or xenogeneic‐derived organoids. The lower panel describes the potential to incorporate those improved organoids in a scaffold, offering the possibility to explore new implantation sites.
Figure 6.
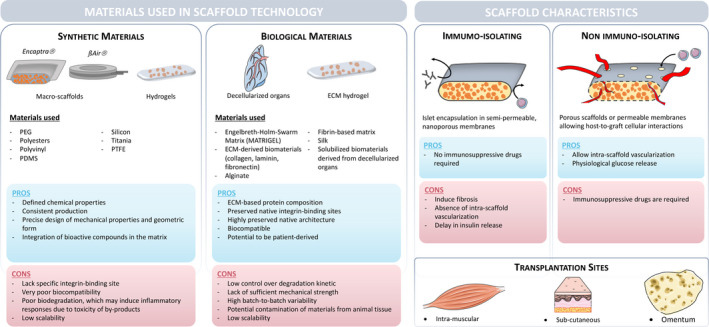
Scaffold generation. The first table shows the types of material available for scaffold generation, divided into synthetic and biological origins, with their advantages and disadvantages. The second table describes, according to scaffold sizes, the type of scaffolds, their advantages and disadvantages, the immunomodulation potentials and the possible sites of transplantation. PEG = polyethylene glycol, PDMS = polydimethylsiloxane, PTFE = polytetrafluoroethylene, ECM = extracellular matrix, MSC = mesenchymal stem cell, and hAEC = human amniotic epithelial cell.
Challenges and perspectives of insulin‐secreting organoids
One of the challenges of ß‐cell replacement is the insulin‐secreting cell source. The high number of human islets needed per recipient in IT, together with the scarcity of available organs, is the main reason why this therapy cannot be proposed to more diabetic patients. Among alternative sources, xeno‐derived primary islet cells have been intensively studied and have even been transplanted into humans without immunosuppression. Despite the persistence of a detectable C‐peptide, none of the trials demonstrated significant graft function [101, 102].
Beta‐cell lines are useful mainly for in vitro investigation but uncontrollable proliferation characteristics limit their translation to clinical practice. Human embryonic stem cells (HESCs) have been successfully differentiated into pancreatic endodermal cells (PEC) and have shown great potential for offering an unlimited source of insulin‐secreting cells [103, 104]. However, PECs take several weeks to mature after transplantation. Recently, ViaCyte performed clinical trials using PECs encapsulated in the Encaptra® Drug Delivery System [105]. However, foreign body reaction hasbeen reported as a limiting factor for the engraftment of encapsulated cells [106].
iPSCs represent another valuable cell source. They can be obtained from the recipient and transplanted without risk of allorejection. However, the transplanted material would still face autoimmune destruction and the personalized production of a sufficient number of iPSCs is a significant challenge in terms of logistics and costs [107]. In addition, hESC and iPSC are associated with a potential risk of teratogenicity that is not well characterized yet, and calls for caution with their use in humans.
We previously highlighted the importance of intercellular communications within the islet and the complexity of this micro‐organ. It may therefore be crucial to re‐establish those connections when engineering organoids. Beta cells generated from hESCs, iPSCs, or transdifferentiation processes are certainly promising cells sources for insulin‐secreting tissue. However, it is important to take other islet cell types, especially α and δ cells, into consideration when generating organoids. It has been demonstrated that the cross talk between different types of islet cells generates inhibitory and stimulatory signals affecting blood glucose homeostasis [108].
With the rapid development of genome editing, the CRISP‐Cas9 or other systems have been used to “humanize” xeno‐derived islets [109], to transfect nonislet cells with glucose‐related promoters to express insulin [110], or to modulate immunity [111].
Another challenge of organoid generation for T1D treatment is managing the large‐scale production in order to obtain the functional mass of tissue required to establish proper metabolic control in one individual, and further, to make this therapy accessible to as many patients as possible. Of all the methods enumerated in this review, the automated hanging drop technique and the use of microwell‐containing culture plates seem to be the most versatile and fit for the necessary upscaling [45]. Automated methods in combination with the use of 3D printing will most likely shape the future of tissue engineering. A key aspect will be the cell sources used for the development of PIs. The use of patient‐derived insulin‐secreting cells (autologous) is very interesting for immunologic reasons. This personalized medicine approach, in which the cell product is tailored to each individual patient as he needs it, is very attractive, but implies substantial costs and logistics.
On the other hand, deriving such constructs from hESCs, adult stem cells or from xenogenic origins would allow the continuous production of an off‐the‐shelf, universal cell product, which could be engineered in a limited number of dedicated, centralized facilities. The direction that ß‐cell replacement will take in the future remains open, but the field has reached a stimulating point, where many opportunities are close to hand, with clear prospects of a breakthrough for cell‐based therapies for T1D.
Conclusion
Organoid generation, with the possibility of incorporating supporting cells to an insulin‐producing construct, represents a valuable strategy to overcome the hurdles faced by islet transplantation. By improving viability, function, and engraftment, the amount of islets required per recipient will be lowered, thus reducing the number of donors needed to achieve full glycemic control. Altogether, and in combination with the development of automated methods for industrial organoid generation, islet transplantation could become accessible as a therapy on a much larger scale. Furthermore, these advances will most likely open the path toward new transplantation sites, allowing to move away from the hostile liver microenvironment currently used.
Allorejection and auto‐immunity recurrence are major issues in the development of islet transplantation. The need for systemic immunosuppression, which puts patients at risk of infection and neoplasia, makes this therapy available only to selected T1D patients with severe disease. The modulation of the immune system, using cells such as MSCs or AECs, or utilizing gene therapy approaches, would potentially allow the reduction or even the elimination of the need for immunosuppressive drugs.
Finally, the use of induced pluripotent stem cells as a substrate for insulin‐producing organoids could resolve the issue of organ shortage [112]. Ultimately, insulin‐producing organoids, constructed with the approaches described in this review, could be used as building blocks for the bioengineering of a larger structure, and represent the first and major step toward the creation of a bioartificial pancreas.
Authorship
CW: designed, researched, and wrote the manuscript. FL and KB: contributed to manuscript contents and writing. TB and DB: critically revised the manuscript. EB: designed and supervised the writing of the manuscript.
Funding
EB is supported by grants from the European Foundation for the Study of Diabetes, Horizon 2020 Framework Program (VANGUARD 874700), Juvenile Diabetes Research Foundation (grant # 3‐SRA‐2020‐926‐S‐B), and the Shota Rustaveli National Science Foundation (FR‐19‐19760); TB and DB are supported by grants from the Swiss National Science Foundation (grant #310030_173138 and #310030_170090).
Conflicts of interest
The authors have no conflict of interest to declare.
References
- 1. International Diabetes Federation (IDF) . Diabetes Atlas ninth edition, Brussels, Belgium, 2019. In: International Diabetes Federation; 2019. Available at: https://www.diabetesatlas.org. Last accessed June 19, 2020. [Google Scholar]
- 2. Stratton IM, Adler AI, Neil HA, et al Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Samoylova ML, Borle D, Ravindra KV. Pancreas transplantation: indications, techniques, and outcomes. Surg Clin North Am 2019; 99: 87. [DOI] [PubMed] [Google Scholar]
- 4. Niclauss N, Meier R, Bédat B, Berishvili E, Berney T. Beta‐cell replacement: pancreas and islet cell transplantation. Endocr Dev 2016; 31: 146. [DOI] [PubMed] [Google Scholar]
- 5. Nyqvist D, Köhler M, Wahlstedt H, Berggren PO. Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes 2005; 54: 2287. [DOI] [PubMed] [Google Scholar]
- 6. Barton FB, Rickels MR, Alejandro R, et al Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 2012; 35: 1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clevers H. Modeling development and disease with organoids. Cell 2016; 165: 1586. [DOI] [PubMed] [Google Scholar]
- 8. Ionescu‐Tirgoviste C, Gagniuc PA, Gubceac E, et al A 3D map of the islet routes throughout the healthy human pancreas. Sci Rep 2015; 5: 14634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol 2013; 75: 155. [DOI] [PubMed] [Google Scholar]
- 10. Pisania A, Weir GC, O'Neil JJ, et al Quantitative analysis of cell composition and purity of human pancreatic islet preparations. Lab Invest 2010; 90: 1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ichii H, Inverardi L, Pileggi A, et al A novel method for the assessment of cellular composition and beta‐cell viability in human islet preparations. Am J Transplant 2005; 5: 1635. [DOI] [PubMed] [Google Scholar]
- 12. Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA 2006; 103: 2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodriguez‐Diaz R, Caicedo A. Neural control of the endocrine pancreas. Best Pract Res Clin Endocrinol Metab 2014; 28: 745. [DOI] [PubMed] [Google Scholar]
- 14. Arrojo e Drigo R, Ali Y, Diez J, Srinivasan DK, Berggren P‐O, Boehm BO. New insights into the architecture of the islet of Langerhans: a focused cross‐species assessment. Diabetologia 2015; 58: 2218. [DOI] [PubMed] [Google Scholar]
- 15. Aamodt KI, Powers AC. Signals in the pancreatic islet microenvironment influence β‐cell proliferation. Diabetes Obes Metab 2017; 19: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Konstantinova I, Nikolova G, Ohara‐Imaizumi M, et al EphA‐Ephrin‐A‐mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell 2007; 129: 359. [DOI] [PubMed] [Google Scholar]
- 17. Esni F, Täljedal IB, Perl AK, Cremer H, Christofori G, Semb H. Neural cell adhesion molecule (N‐CAM) is required for cell type segregation and normal ultrastructure in pancreatic islets. J Cell Biol 1999; 144: 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parnaud G, Lavallard V, Bedat B, et al Cadherin engagement improves insulin secretion of single human β‐cells. Diabetes 2015; 64: 887. [DOI] [PubMed] [Google Scholar]
- 19. Bosco D, Rouiller DG, Halban PA. Differential expression of E‐cadherin at the surface of rat beta‐cells as a marker of functional heterogeneity. J Endocrinol 2007; 194: 21. [DOI] [PubMed] [Google Scholar]
- 20. Parnaud G, Gonelle‐Gispert C, Morel P, et al Cadherin engagement protects human β‐cells from apoptosis. Endocrinology 2011; 152: 4601. [DOI] [PubMed] [Google Scholar]
- 21. Bavamian S, Klee P, Britan A, et al Islet‐cell‐to‐cell communication as basis for normal insulin secretion. Diabetes Obes Metab 2007; 9: 118. [DOI] [PubMed] [Google Scholar]
- 22. Henderson JR, Moss MC. A morphometric study of the endocrine and exocrine capillaries of the pancreas. Q J Exp Physiol 1985; 70: 347. [DOI] [PubMed] [Google Scholar]
- 23. Narayanan S, Loganathan G, Dhanasekaran M, et al Intra‐islet endothelial cell and β‐cell crosstalk: implication for islet cell transplantation. World J Transplant 2017; 7: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alvarez‐Perez JC, Ernst S, Demirci C, et al Hepatocyte growth factor/c‐Met signaling is required for β‐cell regeneration. Diabetes 2014; 63: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nikolova G, Jabs N, Konstantinova I, et al The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell 2006; 10: 397. [DOI] [PubMed] [Google Scholar]
- 26. Phelps EA, Cianciaruso C, Santo‐Domingo J, et al Advances in pancreatic islet monolayer culture on glass surfaces enable super‐resolution microscopy and insights into beta cell ciliogenesis and proliferation. Sci Rep 2017; 7: 45961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaido T, Yebra M, Cirulli V, Montgomery AM. Regulation of human beta‐cell adhesion, motility, and insulin secretion by collagen IV and its receptor alpha1beta1. J Biol Chem 2004; 279: 53762. [DOI] [PubMed] [Google Scholar]
- 28. Riopel M, Krishnamurthy M, Li J, Liu S, Leask A, Wang R. Conditional β1‐integrin‐deficient mice display impaired pancreatic β cell function. J Pathol 2011; 224: 45. [DOI] [PubMed] [Google Scholar]
- 29. Bosco D, Meda P, Halban PA, Rouiller DG. Importance of cell‐matrix interactions in rat islet beta‐cell secretion in vitro: role of alpha6beta1 integrin. Diabetes 2000; 49: 233. [DOI] [PubMed] [Google Scholar]
- 30. Parnaud G, Hammar E, Rouiller DG, Armanet M, Halban PA, Bosco D. Blockade of beta1 integrin‐laminin‐5 interaction affects spreading and insulin secretion of rat beta‐cells attached on extracellular matrix. Diabetes 2006; 55: 1413. [DOI] [PubMed] [Google Scholar]
- 31. Armanet M, Wojtusciszyn A, Morel P, et al Regulated laminin‐332 expression in human islets of Langerhans. FASEB J 2009; 23: 4046. [DOI] [PubMed] [Google Scholar]
- 32. Brissova M, Shostak A, Shiota M, et al Pancreatic islet production of vascular endothelial growth factor–a is essential for islet vascularization, revascularization, and function. Diabetes 2006; 55: 2974. [DOI] [PubMed] [Google Scholar]
- 33. Meyer T, Chodnewska I, Czub S, et al Extracellular matrix proteins in the porcine pancreas: a structural analysis for directed pancreatic islet isolation. Transplant Proc 1998; 30: 354. [DOI] [PubMed] [Google Scholar]
- 34. Van Deijnen JH, Van Suylichem PT, Wolters GH, Van Schilfgaarde R. Distribution of collagens type I, type III and type V in the pancreas of rat, dog, pig and man. Cell Tissue Res 1994; 277: 115. [DOI] [PubMed] [Google Scholar]
- 35. Ricordi C, Alejandro R, Rilo HH, et al Long‐term in vivo function of human mantled islets obtained by incomplete pancreatic dissociation and purification. Transplant Proc 1995; 27: 3382. [PubMed] [Google Scholar]
- 36. Thomas FT, Contreras JL, Bilbao G, Ricordi C, Curiel D, Thomas JM. Anoikis, extracellular matrix, and apoptosis factors in isolated cell transplantation. Surgery 1999; 126: 299. [PubMed] [Google Scholar]
- 37. Carlsson PO, Palm F, Mattsson G. Low revascularization of experimentally transplanted human pancreatic islets. J Clin Endocrinol Metab 2002; 87: 5418. [DOI] [PubMed] [Google Scholar]
- 38. Mattsson G, Jansson L, Carlsson PO. Decreased vascular density in mouse pancreatic islets after transplantation. Diabetes 2002; 51: 1362. [DOI] [PubMed] [Google Scholar]
- 39. Tremmel DM, Odorico JS. Rebuilding a better home for transplanted islets. Organogenesis 2018; 14: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Laschke MW, Menger MD. Life is 3D: boosting spheroid function for tissue engineering. Trends Biotechnol 2017; 35: 133. [DOI] [PubMed] [Google Scholar]
- 41. Achilli TM, Meyer J, Morgan JR. Advances in the formation, use and understanding of multi‐cellular spheroids. Expert Opin Biol Ther 2012; 12: 1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jun Y, Lee J, Choi S, et al In vivo–mimicking microfluidic perfusion culture of pancreatic islet spheroids. Science Advances 2019; 5: eaax4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eder T, Eder IE. 3D Hanging drop culture to establish prostate cancer organoids. Methods Mol Biol 2017; 1612: 167. [DOI] [PubMed] [Google Scholar]
- 44. Kim JH, Lim IR, Joo HJ, et al Sphere formation of adipose stem cell engineered by poly‐2‐hydroxyethyl methacrylate induces in vitro angiogenesis through fibroblast growth factor 2. Biochem Biophys Res Commun 2015; 468: 372. [DOI] [PubMed] [Google Scholar]
- 45. Wassmer CH, Bellofatto K, Perez L, et al Engineering of primary islet cell spheroids for 3D culture or transplantation: a methodological comparative study. Cell Transplant 2020; 29: 963689720937292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tung YC, Hsiao AY, Allen SG, Torisawa YS, Ho M, Takayama S. High‐throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 2011; 136: 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Das T, Meunier L, Barbe L, et al Empirical chemosensitivity testing in a spheroid model of ovarian cancer using a microfluidics‐based multiplex platform. Biomicrofluidics 2013; 7: 11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu L, Chen MC, Cheung KC. Droplet‐based microfluidic system for multicellular tumor spheroid formation and anticancer drug testing. Lab Chip 2010; 10: 2424. [DOI] [PubMed] [Google Scholar]
- 49. Prior N, Inacio P, Huch M. Liver organoids: from basic research to therapeutic applications. Gut 2019; 68: 2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bhang SH, Cho SW, La WG, et al Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose‐derived stromal cells. Biomaterials 2011; 32: 2734. [DOI] [PubMed] [Google Scholar]
- 51. Namba Y, Sogawa C, Okusha Y, et al Depletion of lipid efflux pump ABCG1 triggers the intracellular accumulation of extracellular vesicles and reduces aggregation and tumorigenesis of metastatic cancer cells. Front Oncol 2018; 8: 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lebreton F, Lavallard V, Bellofatto K, et al Insulin‐producing organoids engineered from islet and amniotic epithelial cells to treat diabetes. Nat Commun. 2019; 10: 4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dissanayaka WL, Zhu L, Hargreaves KM, Jin L, Zhang C. In vitro analysis of scaffold‐free prevascularized microtissue spheroids containing human dental pulp cells and endothelial cells. J Endod 2015; 41: 663. [DOI] [PubMed] [Google Scholar]
- 54. Bartosh TJ, Ylöstalo JH, Mohammadipoor A, et al Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci USA 2010; 107: 13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ylöstalo JH, Bartosh TJ, Coble K, Prockop DJ. Human mesenchymal stem/stromal cells cultured as spheroids are self‐activated to produce prostaglandin E2 that directs stimulated macrophages into an anti‐inflammatory phenotype. Stem Cells 2012; 30: 2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hauge‐Evans AC, Squires PE, Belin VD, et al Role of adenine nucleotides in insulin secretion from MIN6 pseudoislets. Mol Cell Endocrinol 2002; 19: 167. [DOI] [PubMed] [Google Scholar]
- 57. Rezania A, Bruin JE, Arora P, et al Reversal of diabetes with insulin‐producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 2014; 32: 1121. [DOI] [PubMed] [Google Scholar]
- 58. Tan J, Liu L, Li B, et al Pancreatic stem cells differentiate into insulin‐secreting cells on fibroblast‐modified PLGA membranes. Mater Sci Eng C Mater Biol Appl 2019; 97: 593. [DOI] [PubMed] [Google Scholar]
- 59. Zhang D, Jiang W, Liu M, et al Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin‐producing cells. Cell Res 2009; 19: 429. [DOI] [PubMed] [Google Scholar]
- 60. Furuyama K, Chera S, van Gurp L, et al Diabetes relief in mice by glucose‐sensing insulin‐secreting human α‐cells. Nature 2019; 567: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Meivar‐Levy I, Ferber S. Reprogramming of liver cells into insulin‐producing cells. Best Pract Res Clin Endocrinol Metab 2015; 29: 873. [DOI] [PubMed] [Google Scholar]
- 62. Halban PA, Powers SL, George KL, Bonner‐Weir S. Spontaneous reassociation of dispersed adult rat pancreatic islet cells into aggregates with three‐dimensional architecture typical of native islets. Diabetes 1987; 36: 783. [DOI] [PubMed] [Google Scholar]
- 63. Matta SG, Wobken JD, Williams FG, Bauer GE. Pancreatic islet cell reaggregation systems: efficiency of cell reassociation and endocrine cell topography of rat islet‐like aggregates. Pancreas 1994; 9: 439. [PubMed] [Google Scholar]
- 64. Dybala MP, Hara M. Heterogeneity of the human pancreatic islet. Diabetes 2019; 68: 1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Giuliani M, Moritz W, Bodmer E, et al Central necrosis in isolated hypoxic human pancreatic islets: evidence for postisolation ischemia. Cell Transplant 2005; 14: 67. [DOI] [PubMed] [Google Scholar]
- 66. Lehmann R, Zuellig RA, Kugelmeier P, et al Superiority of small islets in human islet transplantation. Diabetes 2007; 56: 594. [DOI] [PubMed] [Google Scholar]
- 67. Yu Y, Gamble A, Pawlick R, et al Bioengineered human pseudoislets form efficiently from donated tissue, compare favourably with native islets in vitro and restore normoglycaemia in mice. Diabetologia 2018; 61: 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zuellig RA, Cavallari G, Gerber P, et al Improved physiological properties of gravity‐enforced reassembled rat and human pancreatic pseudo‐islets. J Tissue Eng Regen Med 2017; 11: 109. [DOI] [PubMed] [Google Scholar]
- 69. Lavallard V, Armanet M, Parnaud G, et al Cell rearrangement in transplanted human islets. FASEB J 2016; 30: 748. [DOI] [PubMed] [Google Scholar]
- 70. Kakabadze Z, Gupta S, Pileggi A, et al Correction of diabetes mellitus by transplanting minimal mass of syngeneic islets into vascularized small intestinal segment. Am J Transplant 2013; 13: 2550. [DOI] [PubMed] [Google Scholar]
- 71. Kakabadze Z, Shanava K, Ricordi C, Shapiro AM, Gupta S, Berishvili E. An isolated venous sac as a novel site for cell therapy in diabetes mellitus. Transplantation 2012; 94: 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kakabadze Z, Gupta S, Brandhorst D, Korsgren O, Berishvili E. Long‐term engraftment and function of transplanted pancreatic islets in vascularized segments of small intestine. Transpl Int 2011; 24: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Harata M, Liu S, Promes JA, Burand AJ, Ankrum JA, Imai Y. Delivery of shRNA via lentivirus in human pseudoislets provides a model to test dynamic regulation of insulin secretion and gene function in human islets. Physiol Rep 2018; 6: 13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Teraoku H, Lenzen S. Dynamics of insulin secretion from EndoC‐betaH1 beta‐cell pseudoislets in response to glucose and other nutrient and nonnutrient secretagogues. J Diabetes Res 2017; 2017: 2309630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller‐Klieser W, Kunz‐Schughart LA. Multicellular tumor spheroids: an underestimated tool is catching up again. J Biotechnol. 2010; 148: 3. [DOI] [PubMed] [Google Scholar]
- 76. Weiswald LB, Bellet D, Dangles‐Marie V. Spherical cancer models in tumor biology. Neoplasia 2015; 17: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wojtusciszyn A, Armanet M, Morel P, Berney T, Bosco D. Insulin secretion from human beta cells is heterogeneous and dependent on cell‐to‐cell contacts. Diabetologia 2008; 51: 1843. [DOI] [PubMed] [Google Scholar]
- 78. Penko D, Mohanasundaram D, Sen S, et al Incorporation of endothelial progenitor cells into mosaic pseudoislets. Islets 2011; 3: 73. [DOI] [PubMed] [Google Scholar]
- 79. Urbanczyk M, Zbinden A, Layland SL, Duffy G, Schenke‐Layland K. Controlled heterotypic pseudo‐islet assembly of human β‐cells and human umbilical vein endothelial cells using magnetic levitation. Tissue Eng Part A 2020; 26: 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jiao A, Li F, Zhang C, Lv W, Chen B, Zhang J. Simulated cholinergic reinnervation of beta (INS‐1) cells: antidiabetic utility of heterotypic pseudoislets containing beta cell and cholinergic cell. Int J Endocrinol 2018; 2018: 1505307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jun Y, Kang AR, Lee JS, et al 3D co‐culturing model of primary pancreatic islets and hepatocytes in hybrid spheroid to overcome pancreatic cell shortage. Biomaterials 2013; 34: 3784. [DOI] [PubMed] [Google Scholar]
- 82. Sakata N, Goto M, Yoshimatsu G, Egawa S, Unno M. Utility of co‐transplanting mesenchymal stem cells in islet transplantation. World J Gastroenterol. 2011; 17: 5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Arzouni AA, Vargas‐Seymour A, Dhadda PK, et al Characterization of the effects of mesenchymal stromal cells on mouse and human islet function. Stem Cells Transl Med 2019; 8: 935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ito T, Itakura S, Todorov I, et al Mesenchymal stem cell and islet co‐transplantation promotes graft revascularization and function. Transplantation 2010; 89: 1438. [DOI] [PubMed] [Google Scholar]
- 85. Lazennec G, Jorgensen C. Concise review: adult multipotent stromal cells and cancer: risk or benefit? Stem Cells 2008; 26: 1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhu W, Xu W, Jiang R, et al Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol 2006; 80: 267. [DOI] [PubMed] [Google Scholar]
- 87. Wassmer CH, Berishvili E. Immunomodulatory properties of amniotic membrane derivatives and their potential in regenerative medicine. Curr Diab Rep 2020; 20: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol 2013; 13: 23. [DOI] [PubMed] [Google Scholar]
- 89. Xu H, Zhang J, Tsang KS, Yang H, Gao WQ. Therapeutic potential of human amniotic epithelial cells on injuries and disorders in the central nervous system. Stem Cells Int 2019; 2019: 5432301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Miki T. Stem cell characteristics and the therapeutic potential of amniotic epithelial cells. Am J Reprod Immunol 2018; 80: e13003. [DOI] [PubMed] [Google Scholar]
- 91. Ferreira LMR, Meissner TB, Tilburgs T, Strominger JL. HLA‐G: at the interface of maternal‐fetal tolerance. Trends Immunol 2017; 38: 272. [DOI] [PubMed] [Google Scholar]
- 92. Lebreton F, Bellofatto K, Wassmer CH, et al Shielding islets with human amniotic epithelial cells enhances islet engraftment and revascularization in a murine diabetes model. Am J Transplant 2020; 20: 1551. [DOI] [PubMed] [Google Scholar]
- 93. Lebreton F, Lavallard V, Bellofatto K, et al Insulin‐producing organoids engineered from islet‐ and amniotic epithelial cells to treat diabetes. Nat Commun 2019; 10: 4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Qureshi KM, Lee J, Paget MB, et al Low gravity rotational culture and the integration of immunomodulatory stem cells reduce human islet allo‐reactivity. Clin Transplant 2015; 29: 90. [DOI] [PubMed] [Google Scholar]
- 95. Zafar A, Lee J, Yesmin S, et al Rotational culture and integration with amniotic stem cells reduce porcine islet immunoreactivity in vitro and slow xeno‐rejection in a murine model of islet transplantation. Xenotransplantation 2019; 26: e12508. [DOI] [PubMed] [Google Scholar]
- 96. Hunckler MD, García AJ. Engineered biomaterials for enhanced function of insulin‐secreting β‐cell organoids. Adv Funct Mater 2020: 2000134 [Epub ahead of print] 10.1002/adfm.202000134 [DOI] [Google Scholar]
- 97. Desai T, Shea LD. Advances in islet encapsulation technologies. Nat Rev Drug Discov 2017; 16: 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sackett SD, Tremmel DM, Ma F, et al Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas. Sci Rep 2018; 8: 10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Citro A, Moser PT, Dugnani E, et al Biofabrication of a vascularized islet organ for type 1 diabetes. Biomaterials 2019; 199: 40. [DOI] [PubMed] [Google Scholar]
- 100. Orive G, Emerich D, Khademhosseini A, et al Engineering a clinically translatable bioartificial pancreas to treat type I diabetes. Trends Biotechnol 2018; 36: 445. [DOI] [PubMed] [Google Scholar]
- 101. Groth CG, Korsgren O, Tibell A, et al Transplantation of porcine fetal pancreas to diabetic patients. Lancet 1994; 344: 1402. [DOI] [PubMed] [Google Scholar]
- 102. Matsumoto S, Tan P, Baker J, et al Clinical porcine islet xenotransplantation under comprehensive regulation. Transplant Proc 2014; 46: 1992. [DOI] [PubMed] [Google Scholar]
- 103. Kubo A, Shinozaki K, Shannon JM, et al Development of definitive endoderm from embryonic stem cells in culture. Development 2004; 131: 1651. [DOI] [PubMed] [Google Scholar]
- 104. D'Amour KA, Bang AG, Eliazer S, et al Production of pancreatic hormone‐expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 2006; 24: 1392. [DOI] [PubMed] [Google Scholar]
- 105. https://viacyte.com/clinical‐trials/. Last accessed June 19, 2020.
- 106. Henry RPJ, Wilensky J, Shapiro AMJ, et al Initial clinical evaluation of VC‐01TM combination Product‐A stem cell‐derived islet replacement for type 1 diabetes (T1D). Diabetes 2018; 67: 138. [Google Scholar]
- 107. Gamble A, Pepper AR, Bruni A, Shapiro AMJ. The journey of islet cell transplantation and future development. Islets 2018; 10: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Jo J, Choi MY, Koh DS. Beneficial effects of intercellular interactions between pancreatic islet cells in blood glucose regulation. J Theor Biol 2009; 257: 312. [DOI] [PubMed] [Google Scholar]
- 109. Mourad NI, Gianello P. Gene editing, gene therapy, and cell xenotransplantation: cell transplantation across species. Curr Transplant Rep 2017; 4: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gan SU, Notaridou M, Fu ZY, et al Correction of murine diabetic hyperglycaemia with a single systemic administration of an AAV2/8 vector containing a novel codon optimized human insulin gene. Curr Gene Ther 2016; 16: 65. [DOI] [PubMed] [Google Scholar]
- 111. Li D, Zhao B, Luo Y, et al Transplantation of Aire‐overexpressing bone marrow‐derived dendritic cells delays the onset of type 1 diabetes. Int Immunopharmacol 2017; 49: 13. [DOI] [PubMed] [Google Scholar]
- 112. Sneddon JB, Tang Q, Stock P, et al Stem cell therapies for treating diabetes: progress and remaining challenges. Cell Stem Cell 2018; 22: 810. [DOI] [PMC free article] [PubMed] [Google Scholar]


