Figure 3.
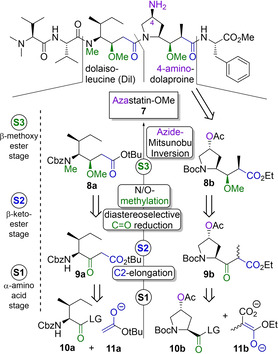
Retrosynthetic analysis of the target molecule. Both of the key γ‐amino acids dolaisoleucine and 4‐aminodolaproine were to be obtained from their respective α‐amino acid homologues (stage 1) in an analogous fashion: C2‐elongation was to yield β‐keto esters (stage 2), which were to be reduced diastereoselectively to furnish highly chiral β‐methoxy esters (stage 3).
