Abstract
Objective
To determine the onset of preventive efficacy with eptinezumab in patients with migraine.
Background
Eptinezumab is a monoclonal antibody inhibiting calcitonin gene‐related peptide approved as an intravenously administered treatment for the prevention of migraine.
Methods
Patients who received eptinezumab 100 mg, eptinezumab 300 mg, or placebo in PROMISE7‐1 (episodic migraine; 100 mg, n = 221; 300 mg, n = 222; placebo, n = 222) or PROMISE7‐2 (chronic migraine; 100 mg, n = 356; 300 mg, n = 350; placebo, n = 366) were included. Testing of the percentage of patients with a migraine on day 1 after dosing was prespecified and alpha‐controlled. In further exploration of this prespecified endpoint, a post hoc closed testing procedure, which controlled the false‐positive (type 1) error rate, provided a statistically rigorous evaluation of migraine prevention onset. The procedure involved up to 84 tests of significance, all of which were performed in sequence until the first nonsignificant result.
Results
For both studies, all tests for significance for eptinezumab 100 and 300 mg, from days 1‐84 through day 1 alone, achieved nominal significance (P < .05), indicating that eptinezumab was fully effective beginning on day 1. Over each interval, the treatment effect was comparable to the effect over weeks 1‐12. Mean changes from baseline in monthly migraine days for the primary endpoint period ranged from −3.9 to −4.9, −4.1 to −4.9, and −2.2 to −3.2 for eptinezumab 100, 300 mg, and placebo, respectively, in PROMISE7‐1 and from −7.2 to −8.0, −7.9 to −8.2, and −4.3 to −5.6, respectively, in PROMISE7‐2. The difference from placebo (95% confidence interval) in day 1 treatment effect was −2.2 (−4.1, −0.3) and −2.5 (−4.4, −0.6) days/month for eptinezumab 100 and 300 mg, respectively, in PROMISE7‐1, and was −3.8 (−5.6, −2.0) and −4.0 (−5.8, −2.1) days/month for 100 and 300 mg, respectively, in PROMISE7‐2.
Conclusions
The migraine preventive effect of eptinezumab is rapid and sustained in patients with episodic or chronic migraine, with onset of optimal preventive efficacy observed on the day following the initial dose.
Keywords: eptinezumab, monoclonal antibody, episodic migraine, chronic migraine, onset of efficacy
Introduction
Patients with migraine consider speed of onset as one of the most important attributes of preventive treatment, second to efficacy. 1 Patients’ decisions to discontinue or switch preventive medication can be made as early as 30 days after beginning treatment; 2 , 3 thus, there is a need for preventive treatments with a rapid onset of sustained effect to ensure patient adherence, management of acute medications, and beneficial long‐term outcomes. Unfortunately, commonly prescribed preventive migraine therapies can take 2‐6 months to achieve maximal effects. 4 , 5 , 6 Although data from most newer agents suggest that they may have earlier onset, the time to clinically meaningful and optimal benefit remains undefined 7 , 8 , 9 , 10 , 11 , 12 , 13 and a statistically robust methodology to determine onset of full efficacy of a preventive agents has, to date, not been determined.
Eptinezumab, a humanized calcitonin gene‐related peptide (CGRP)‐targeted monoclonal antibody (mAb), was recently approved by US Food and Drug Administration for the preventive treatment of migraine in adults. Eptinezumab was developed to provide an early onset of preventive action that is sustained throughout a 12‐week dosing interval. By intravenous (IV) administration over 30 minutes, eptinezumab has 100% bioavailability and achieves maximum plasma concentration at the end of IV administration. 14 Pharmacokinetic results coupled with data from phase 2 studies 15 , 16 suggested that migraine preventive efficacy of eptinezumab may begin immediately, leading to the inclusion of the percentage of patients reporting a migraine headache on day 1 as a prespecified and alpha‐controlled endpoint in pivotal phase 3 trials conducted in patients with episodic (PROMISE7‐1) and chronic (PROMISE7‐2) migraine. In both pivotal trials, the percentage of patients reporting a migraine on day 1 was reduced by more than 50% compared to baseline in eptinezumab‐treated patients and by approximately 25% in placebo patients. 17 , 18 However, questions remained if the results on this specific day (day 1) represented onset of benefit or a transient benefit; we hypothesized that day 1 represented the onset of treatment. The objective of this post hoc closed testing analysis was to determine whether this day 1 observation represents the point at which the preventive benefit of eptinezumab was established and whether that effect was sustained without interruption for 12 weeks in this broad spectrum of patients with episodic and chronic migraine. This methodology has the potential to better align patient expectations and the capabilities of drug development.
Methods
Study Overview and Design
PROMISE7‐1 (ClinicalTrials.gov: NCT02559895) 17 and PROMISE7‐2 (ClinicalTrials.gov: NCT02974153) 18 were randomized, double‐blind, parallel‐group, and placebo‐controlled trials of eptinezumab for the preventive treatment of migraine. Both studies were conducted in accordance with International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use guideline E6, local regulatory requirements, and the principles of the Declaration of Helsinki. The independent ethics committee or institutional review board for each study site reviewed and approved the study protocol. All patients provided written informed consent prior to their study participation.
PROMISE7‐1 evaluated up to 4 IV doses of eptinezumab 30, 100, 300 mg, or placebo administered every 12 weeks in patients with episodic migraine, and PROMISE7‐2 evaluated 2 IV doses of eptinezumab 100, 300 mg, or placebo administered every 12 weeks in patients with chronic migraine. The sample sizes for these studies (200 patients per arm for PROMISE7‐1 and 350 patients per arm for PROMISE7‐2) were driven by power calculations associated with the primary endpoint for each study. The planned sample size provided at least 90% power for the primary endpoint and at least 90% power for the key secondary day 1 endpoint, for a treatment difference of at least 15 percentage points with respect to the percentage of patients with a migraine on day 1, which, when extrapolated to 28 days, corresponds to a difference of 4.2 monthly migraine days (MMDs). Because the 30‐mg dose in PROMISE7‐1 did not achieve statistical significance in any measure per the prespecified testing hierarchy, data from the 30‐mg dose were not included in this closed testing analysis. Both PROMISE7‐1 and PROMISE7‐2 included a 28‐day screening period; only patients completing an eDiary on ≥25 days of the 28‐day screening period in PROMISE7‐1 or ≥24 days of the 28‐day screening period in PROMISE7‐2 were eligible for study participation. The primary efficacy endpoint in both PROMISE7‐1 and PROMISE7‐2 was the mean change from baseline in MMDs over weeks 1‐12 (days 1‐84), assessed using eDiary data.
Statistical Analyses
Migraine prevention studies rely upon reductions in MMDs to demonstrate efficacy. 10 , 13 , 19 , 20 , 21 Historically, this metric has been sufficient to evaluate onset of benefit, as it can take a month or more. Because the onset of eptinezumab efficacy was hypothesized to occur within a day, reductions in monthly migraine frequency are not directly useful in determining onset for eptinezumab; instead, the presence or absence of a migraine on specific days was selected as the measurement tool. The PROMISE7‐2 protocol specified this presence or absence of a migraine on the day following the infusion (day 1) as the metric to determine if treatment onset occurred by day 1. To summarize this measure over the treatment arms, we used the percentage of patients reporting a migraine day. When extending this measure to multiple days, we calculated the average percentage across the time period (eg, the average percentage of patients with a migraine on days 1‐4), which is equivalent to the average percentage of days a patient had a migraine during that time period (eg, for each patient, determine what percentage of these days [days 1‐4] they had a migraine, and average that across patients). To allow for comparisons to MMDs, the above measures were normalized to a 28‐day month, which corresponds to the number of migraine days a patient would be expected to have over 28 days if the rate observed was continued for the entire 28‐day period (eg, if a patient had a migraine on one of days 1‐4, which corresponds to 25% of days, then, with a similar pattern over 28 days, it would be expected that the patient would experience 7 MMDs).
The normalized MMD endpoint employed the same missing data imputation algorithm as was used for the primary endpoint, which was described in detail in the primary reports. 17 , 18 If a patient failed to complete the diary on a given day, it was assumed the migraine rate on that day was identical to the rate seen for the non‐missing days in that month. If a patient failed to complete the diary on more than 7 days in a month, a weighted estimated rate based upon the current month and the prior month was used to determine what rate to impute.
In the current analysis, the timing of treatment onset was determined by finding the first day from which a treatment effect was nominally significant and sustained without interruption through the end of the first dosing interval (week 12). To ensure the method used was statistically valid with multiplicity control, a closed testing procedure was used on ever smaller time intervals beginning with the primary endpoint interval. 22 Specifically, the treatment effect was determined and tested over the primary endpoint interval of weeks 1‐12 (days 1‐84). Because statistical significance was known to be present over this interval, the algorithm moved to the next smaller interval, days 1‐83. For each data point in which nominal significance was found (ie, P < .05), the interval was made smaller by 1 day (eg, days 1‐82) and the treatment effect was determined and tested again. For each interval, mean MMDs was calculated as (number of migraine days in the interval × 28)/number of days in the interval. At the first point, in which nominal significance was not achieved (ie, first interval where P > .05), the procedure was stopped, and onset was declared based upon the end day of the prior interval. As an example, should each of the 78 tests from days 1‐84 to 1‐7 result in P < .05 but the result over days 1‐6 not be significant, day 7 would be determined as the onset day because each interval from days 1‐7 to the primary endpoint showed a treatment effect.
The P values presented are 2‐sided and are based upon an ANCOVA model that mirrored the ANCOVA models used for the primary endpoint. These models used change in normalized MMDs from baseline to the time interval of interest as the response variable and used treatment, baseline MMDs, and use of other preventive treatment (PROMISE7‐2 only) as predictor variables. The statistics provided are the least square means for the treatment comparisons.
Data Availability Statement
The data reported are part of an ongoing, global sponsor‐led clinical development and registration program. De‐identified participant data are not available for legal and ethical reasons.
Results
Patients
A total of 888 patients with episodic migraine received treatment in PROMISE7‐1, with 665 patients included in this analysis (eptinezumab 30 mg was not included); 221 received eptinezumab 100 mg, 222 received eptinezumab 300 mg, and 222 received placebo in the full analysis population. 17 The mean patient age was 39.8 years and the mean number of MMDs during the 28‐day screening period was approximately 8.6 across treatment groups. This represents an average daily probability of having a migraine of 0.31 (or 31%).
A total of 1072 patients with chronic migraine received treatment in PROMISE7‐2; 356 received eptinezumab 100 mg, 350 received eptinezumab 300 mg, and 366 received placebo. 18 The mean patient age was 40.5 years and the mean number of MMDs during the 28‐day screening period was approximately 16.1 across treatment groups. This represents an average daily probability of having a migraine of 0.58 (or 58%).
Baseline demographics and clinical characteristics of patients who received eptinezumab 100 mg, 300 mg, or placebo in either study are summarized in Table 1.
Table 1.
Baseline Demographics and Clinical Characteristics (Safety Population)
| PROMISE7‐1 (Episodic Migraine) | PROMISE7‐2 (Chronic Migraine) | |||||
|---|---|---|---|---|---|---|
| Eptinezumab 100 mg (n = 223) | Eptinezumab 300 mg (n = 224) | Placebo (n = 222) | Eptinezumab 100 mg (n = 356) | Eptinezumab 300 mg (n = 350) | Placebo (n = 366) | |
| Mean (SD) age, years | 40.0 (10.7) | 40.2 (11.7) | 39.9 (11.7) | 41.0 (11.7) | 41.0 (10.4) | 39.6 (11.3) |
| Mean (SD) BMI, kg/m2 | 29.4 (7.6) | 28.9 (7.1) | 29.6 (7.3) | 26.4 (5.0) | 26.2 (5.0) | 27.0 (5.6) |
| Sex, n (%) female | 179 (80.3) | 199 (88.8) | 186 (83.8) | 307 (86.2) | 314 (89.7) | 325 (88.8) |
| Race, n (%) | ||||||
| White | 196 (87.9) | 187 (83.5) | 181 (81.5) | 332 (93.3) | 322 (92.0) | 321 (87.7) |
| Black/African American | 17 (7.6) | 27 (12.1) | 30 (13.5) | 21 (5.9) | 23 (6.6) | 38 (10.4) |
| Asian | 1 (<1) | 1 (<1) | 2 (<1) | 1 (<1) | 1 (<1) | 1 (<1) |
| American Indian/Alaska Native | 0 | 2 (<1) | 1 (<1) | 1 (<1) | 1 (<1) | 1 (<1) |
| Native Hawaiian/Pacific Islander | 1 (<1) | 1 (<1) | 1 (<1) | 0 | 1 (<1) | 0 |
| Multiple races | 7 (3.1) | 5 (2.2) | 5 (2.3) | 1 (<1) | 2 (<1) | 4 (1.1) |
| Other | 1 (<1) | 1 (<1) | 2 (<1) | 0 | 0 | 1 (<1) |
| Mean years since migraine diagnosis | 17.4 | 18.2 | 16.9 | 18.3 | 19.0 | 17.0 |
| Mean (SD) migraine days/month† | 8.7 (2.8) | 8.6 (2.9) | 8.4 (2.7) | 16.1 (4.6) | 16.1 (4.8) | 16.2 (4.6) |
| Mean (SD) headache days/month† | 10.0 (3.0) | 10.1 (3.1) | 9.9 (2.8) | 20.4 (3.1) | 20.4 (3.2) | 20.6 (3.0) |
eDiary‐reported migraine and headache characteristics during the 28‐day screening period.
BMI = body mass index; SD = standard deviation.
Migraine Frequency
In both studies, eptinezumab 100 and 300 mg demonstrated statistically significantly greater improvement in the frequency of migraine days from weeks 1‐12 (days 1‐84) vs placebo, with patients with episodic migraine experiencing approximately 4 fewer MMDs vs baseline and patients with chronic migraine experiencing 8 fewer MMDs vs baseline during the first dosing interval, compared with 3 and 6 fewer MMDs, respectively, with placebo (Fig. 1). Reductions in MMDs remained numerically greater with eptinezumab than with placebo throughout the duration of each study. 17 , 18
Fig. 1.
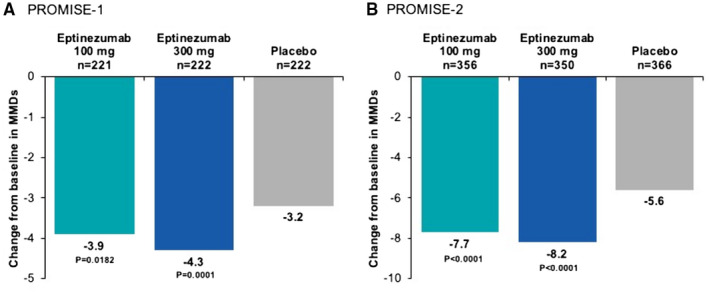
Mean change from baseline in monthly migraine days (MMDs) over weeks 1‐12 (primary endpoint) in (A) PROMISE7‐1 and (B) PROMISE7‐2. Data originally published in Ashina M, et al, 2020 (PROMISE7‐1) 17 and Lipton RB, et al, 2020 (PROMISE7‐2). 18
Percentage of Patients With Migraine on First Day After Dosing
Over the 28‐day baseline period in PROMISE7‐1, the average percentage of patients experiencing a migraine day on any given day was 31.0% in the eptinezumab 100‐mg group, 30.8% in the eptinezumab 300‐mg group, and 29.8% in the placebo group. Multiplying these percentages by 28 days represents the average of 8.7, 8.6, and 8.3 MMDs, respectively. The percentages of patients with a migraine on the first day after dosing were 14.8% in the eptinezumab 100‐mg group (unadjusted P = .031), 13.9% in the eptinezumab 300‐mg group (unadjusted P = .016), and 22.5% in the placebo group; while the P values were <.05, these results were not statistically significant after adjustment for multiplicity. These percentages translate to an average of 4.1, 3.9, and 6.3 MMDs, respectively. Therefore, the percent reductions from baseline are 52.3% (4.6 MMDs), 54.9% (4.7 MMDs), and 24.5% (2.1 MMDs), respectively (Fig. 2A).
Fig. 2.
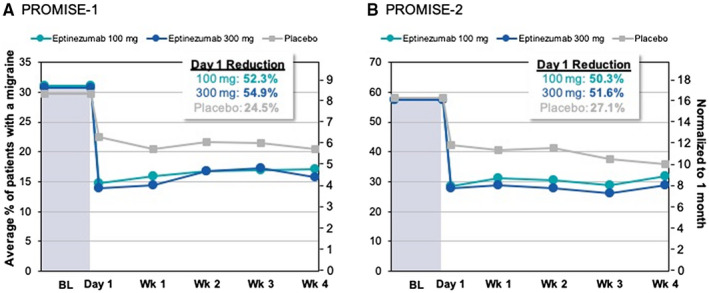
Average daily percentage of patients experiencing migraine in (A) PROMISE7‐1 and (B) PROMISE7‐2. Values for weeks (wks) 1 through 4 calculated as the average daily percentage of patients with a migraine during that week. Normalization to average monthly days was achieved by multiplying the daily percent by 28 days. Data for PROMISE7‐2 originally published in Lipton RB, et al, 2020 (PROMISE7‐2). 18 Baseline (BL, average over the 28‐day screening period).
During the 28 days of screening in PROMISE7‐2, the average percentage of patients experiencing a migraine on any given day was 57.5% in the eptinezumab 100‐mg group, 57.4% in the eptinezumab 300‐mg group, and 58.0% in the placebo group, representing 16.1, 16.1, and 16.2 MMDs, respectively, when multiplied by 28 days. The percentages of patients with a migraine on the first day after dosing were 28.6% in the eptinezumab 100‐mg group (P < .001), 27.8% in the eptinezumab 300‐mg group (P < .001), and 42.3% in the placebo group; these reductions were statistically significant after adjusting for multiplicity. These percentages translate to 8.0, 7.8, and 11.8 MMDs, respectively, and represent reductions from baseline of 50.3% (8.1 MMDs), 51.6% (8.3 MMDs), and 27.1% (4.4 MMDs), respectively (Fig. 2B).
Closed Testing From Day 84 to Day 1
All tests from days 1‐84 through day 1 alone in both studies and for both doses achieved nominal significance, indicating that eptinezumab was effective beginning on day 1 and throughout the entire dosing interval. Figure 3 illustrates the results of the closed testing procedure for PROMISE7‐1 and PROMISE7‐2. The top of each graph presents the mean MMDs for each treatment group. Notably, the curves are very stable on the left, but become more variable on the right; this is because each increment in the curves represents removal of 1 day, which has less influence when going from longer intervals (days 1‐84 to 1‐83) vs shorter intervals (days 1‐2 to day 1). However, the reductions from baseline for both doses of eptinezumab were greater than the reduction for placebo across the entire curve. In addition, the P values for each analysis are presented as circles near the bottom of the graph. As shown, every P value for both studies was <.05. In PROMISE7‐1, the mean change from baseline in MMDs for the primary endpoint period ranged from –3.9 to –4.9 for eptinezumab 100 mg, from –4.1 to –4.9 for eptinezumab 300 mg, and from –2.2 to –3.2 for placebo across the dosing interval. In PROMISE7‐2, the mean change from baseline in MMDs ranged from –7.2 to –8.0 for eptinezumab 100 mg, from –7.9 to –8.2 for eptinezumab 300 mg, and from –4.3 to –5.6 for placebo across the dosing interval. At pivotal moments during the first dosing interval (weeks 1‐4, week 1, days 1‐3, and day 1), eptinezumab demonstrated similar reductions from baseline, with placebo consistently demonstrating smaller reductions (Table 2; Fig. 4). These reductions were similar to the results of the prespecified primary and key secondary efficacy endpoints.
Fig. 3.
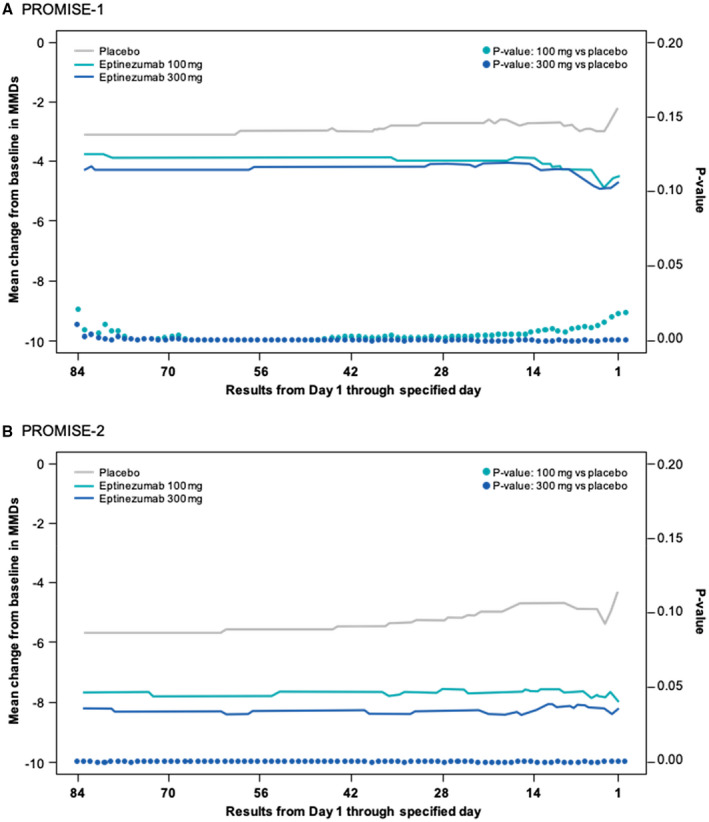
Closed testing evaluation of the onset of effect in (A) PROMISE#x2010;1 and (B) PROMISE7#x2010;2.
Table 2.
Closed Testing Evaluation of the Onset of Effect for Select Intervals (Full Analysis Population)
| Change From Baseline in MMDs | PROMISE7‐1 (Episodic Migraine) | PROMISE7‐2 (Chronic Migraine) | ||||
|---|---|---|---|---|---|---|
| Eptinezumab 100 mg (n = 221) | Eptinezumab 300 mg (n = 222) | Placebo (n = 222) | Eptinezumab 100 mg (n = 356) | Eptinezumab 300 mg (n = 350) | Placebo (n = 366) | |
| Days 1‐84 (Weeks 1‐12) | ||||||
| Estimate mean | −3.9 | −4.3 | −3.2 | −7.7 | −8.2 | −5.6 |
| Mean difference from placebo | −0.7 | −1.1 | −2.0 | −2.6 | ||
| 95% CI | −1.3, −0.1 | −1.7, −0.5 | −2.9, −1.2 | −3.5, −1.7 | ||
| P value | .018 | <.001 | <.001 | <.001 | ||
| Days 1‐28 (Weeks 1‐4) | ||||||
| Estimate mean | −4.0 | −4.1 | −2.8 | −7.5 | −8.2 | −5.2 |
| Mean difference from placebo | −1.2 | −1.4 | −2.3 | −3.1 | ||
| 95% CI | −1.9, −0.5 | −2.0, −0.7 | −3.2, −1.4 | −4.0, −2.2 | ||
| P value | <.001 | <.001 | <.001 | <.001 | ||
| Days 1‐7 (Week 1) | ||||||
| Estimate mean | −4.1 | −4.5 | −2.9 | −7.2 | −7.9 | −4.6 |
| Mean difference from placebo | −1.3 | −1.7 | −2.6 | −3.3 | ||
| 95% CI | −2.2, −0.3 | −2.6, −0.7 | −3.7, −1.5 | −4.4, −2. | ||
| P value | .009 | .001 | <.001 | <.001 | ||
| Days 1‐3 | ||||||
| Estimate mean | −4.9 | −4.9 | −3.0 | −7.9 | −8.2 | −5.4 |
| Mean difference from placebo | −1.9 | −1.9 | −2.5 | −2.8 | ||
| 95% CI | −3.1, −0.6 | −3.1, −0.6 | −3.9, −1.2 | −4.2, −1.5 | ||
| P value | .004 | .004 | <.001 | <.001 | ||
| Days 1‐2 | ||||||
| Estimate mean | −4.6 | −4.9 | −2.6 | −7.7 | −8.4 | −5.0 |
| Mean difference from placebo | −2.0 | −2.3 | −2.8 | −3.4 | ||
| 95% CI | −3.5, −0.6 | −3.7, −0.8 | −4.3, −1.2 | −4.9, −1.9 | ||
| P value | .007 | .002 | <.001 | <.001 | ||
| Day 1 | ||||||
| Estimate mean | −4.5 | −4.7 | −2.2 | −8.0 | −8.2 | −4.3 |
| Mean difference from placebo | −2.2 | −2.5 | −3.8 | −4.0 | ||
| 95% CI | −4.1, −0.3 | −4.4, −0.6 | −5.6, –2.0 | −5.8, −2.1 | ||
| P value | .021 | .010 | <.001 | <.001 | ||
CI = confidence interval; MMDs = monthly migraine days.
Fig. 4.
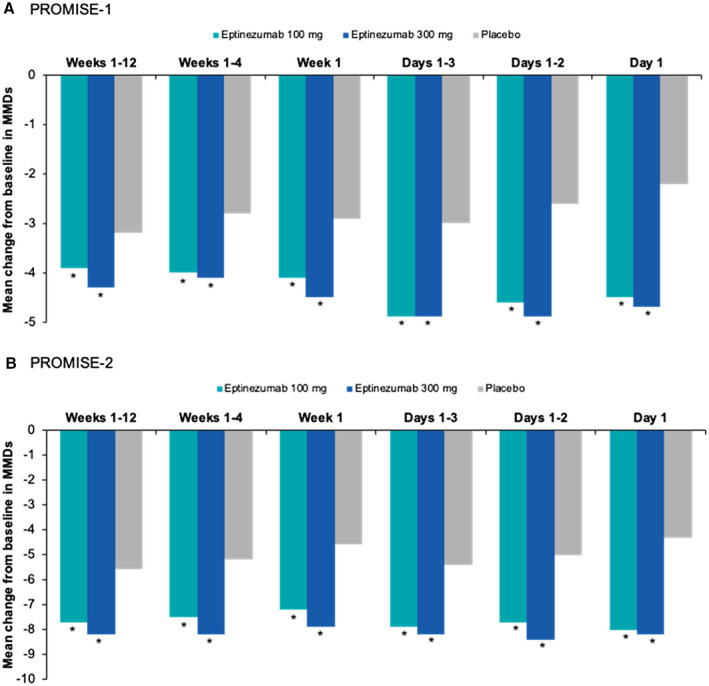
Closed testing evaluation of the onset of effect for select intervals in (A) PROMISE7‐1 and (B) PROMISE7‐2. *P < .05 vs placebo (nominal).
Discussion
In the phase 3 studies for eptinezumab, a prespecified endpoint was included – unique to preventive migraine studies – to capture the percentage of patients with a migraine headache on day 1 after initial dosing. Here, we demonstrate evidence that the maximal preventive treatment effect of eptinezumab can be observed as early as 1 day after IV administration and is sustained for 12 weeks. This closed testing analysis of the PROMISE studies further characterizes the preventive effect of eptinezumab observed in the primary PROMISE reports 17 , 18 – that is, in addition to the onset of a preventive effect observed on day 1, the onset of the optimal treatment effect may be seen as early as the first day after the administration of the treatment. Working backward from the primary endpoint (weeks 1‐12 [days 1‐84]), nominally significant differences vs placebo were observed for every interval tested through day 1 alone. When the percentage of patients with a migraine on any given day in the study, beginning on day 1 after the initial dose, was normalized to average MMDs, the result corresponded to the primary efficacy analysis of reduction in MMDs during weeks 1‐12. Thus, the suppression of CGRP biology in patients with migraine can start within the day after the IV dose and be sustained over the entire 12‐week dosing interval.
The high binding affinity and slow dissociation of the eptinezumab mAb, 14 , 23 , 24 combined with its mean terminal elimination half‐life of 27 days, allow eptinezumab to be delivered every 12 weeks. 14 Eptinezumab is delivered by a 30‐minute IV administration with rapid attainment of maximum plasma concentration by the end of IV administration. 14
Based on the generally delayed onset of traditional migraine preventive therapies and variability in time required to receive maximal benefit, current treatment guidelines for migraine prevention recommend 2 to 6 months to establish efficacy in clinical practice. 6 , 25 In addition, most oral preventive treatment options require gradual titration to a target dose over weeks or months in an effort to minimize side effects. After the gradual onset of efficacy once a target dose is achieved, it may take an additional 2 to 6 months to achieve maximal efficacy. These are reasons why clinical trials of preventive migraine treatments do not typically prespecify endpoints with early time points. In randomized, placebo‐controlled trials of topiramate, onset was defined as the earliest monthly time point when a statistically significant difference was detected that persisted through the remainder of the study, which was found to be month 1 of treatment. 26 , 27 , 28 The primary endpoint for the onabotulinumtoxinA pivotal trials was at week 24, with no prespecified secondary endpoint attempting to determine earliest time to onset of benefit. 29 With the recent emergence of subcutaneously injected CGRP mAbs, the majority of prespecified endpoints in the phase 3 trials for the preventive treatment of episodic and chronic migraine were captured 3‐6 months after treatments were initiated. 10 , 13 , 19 , 20 , 21 , 30 Although these subcutaneously injected CGRP mAbs have published evidence of early onset (within the first week) of migraine preventive effect by calculating separation from placebo in post hoc analyses, 9 , 19 , 31 , 32 , 33 , 34 only one (galcanezumab) has published data from a post hoc closed testing sequential analysis similar to the one used herein. 35 Unlike the eptinezumab analysis, the galcanezumab analysis pooled active doses and used data from studies limited to patients with episodic migraine. Additionally, the intervals used in the galcanezumab analysis were monthly, then each week within the first month, and then days within the first week. Because the eptinezumab analysis examined daily effect from day 84 to day 1, it not only demonstrates the early onset of preventive effect, but also the sustained preventive effect of each dose.
It is reasonable to expect that the early onset of effect could also improve adherence to treatment, as lack of perceived effect is a common reason for preventive therapy discontinuation. 1 In the Second International Burden of Migraine, 24% of patients with episodic migraine and 41% of patients with chronic migraine discontinued preventive therapy (time to discontinuation undefined), with 37‐48% of these premature discontinuations being attributed to lack of efficacy. 3 Data from a more recent (2017) retrospective claims analysis suggest that discontinuation rates may be even higher, with 50% of patients prescribed oral preventive therapies discontinuing within 60 days of initiation, increasing to approximately 75% by 6 months and 85% by 12 months. 2 Furthermore, the evidence of a rapid and sustained effect in the current study could provide important reassurance to patients who fear that any initial response might be fleeting. The maintenance of effect over the long term could potentially improve adherence with therapy as well. Adherence rates in observational studies of oral preventive medication use indicate that adherence generally decreases over time, with only 35‐56% of patients adhering to prescribed regimens at 12 months. 36 Studies designed to evaluate the impact of the early onset of preventive treatments on daily life are needed.
Study Strengths and Limitations
The alpha‐controlled day 1 endpoint to assess efficacy onset in both phase 3 PROMISE trials was the only prespecified endpoint evaluating the time of efficacy onset as the day after initial treatment in migraine prevention trials. The closed testing procedure used to support this endpoint was defined post hoc; therefore, the results must be considered preliminary due to the limitations inherent in post hoc analyses. However, the findings are strengthened by the fact that they are post hoc analyses of predefined endpoints using predefined methodology and that P < .05 had to be repeatedly achieved, thus, controlling for multiplicity – that is, this method tests the null hypothesis Hi if and only if all preceding hypotheses have also been rejected. Like all migraine preventive trials, the sustained response over 12 weeks is based on the population response. Future analyses evaluating individual patient‐level responses over time are necessary to determine the clinical relevance of these findings for an individual patient.
Conclusion
In patients with episodic or chronic migraine, the migraine preventive effect of eptinezumab is rapid, with efficacy observed as early as day 1 after dosing, and sustained, with a similar magnitude of efficacy observed across the treatment period of 84 days. This rapid preventive benefit fulfills an important and unmet treatment need among many patients with migraine, as well as could improve adherence to treatment, patient satisfaction, and suppression of migraine activity to a greater degree than seen with currently available therapies for episodic and chronic migraine. Moreover, the statistical analysis described in this article potentially offers the opportunity to add a new and meaningful dimension to defining successful preventive medications.
Statement of Authorship
Category 1
(a) Conception and Design
Roger Cady, Joe Hirman, Jeff Smith, Steve Snapinn
(b) Acquisition of Data
David W. Dodick, Christopher Gottschalk
(c) Analysis and Interpretation of Data
Joe Hirman, Steve Snapinn
Category 2
(a) Drafting the Manuscript
Roger Cady, Joe Hirman, Steve Snapinn
(b) Revising It for Intellectual Content
David W. Dodick, Christopher Gottschalk, Roger Cady, Joe Hirman, Jeff Smith, Steve Snapinn
Category 3
(a) Final Approval of the Completed Manuscript
David W. Dodick, Christopher Gottschalk, Roger Cady, Joe Hirman, Jeff Smith, Steve Snapinn
Acknowledgments
The authors thank the patients, their families, and the sites that participated in this study. The authors also thank Mary Tom, PharmD, and Nicole Coolbaugh, CMPP, of The Medicine Group, LLC (New Hope, PA, United States) for providing medical writing support, which was funded by H. Lundbeck A/S (Copenhagen, Denmark) in accordance with Good Publication Practice guidelines.
References
- 1. Peres MF, Silberstein S, Moreira F, et al. Patients' preference for migraine preventive therapy. Headache. 2007;47:540-545. [DOI] [PubMed] [Google Scholar]
- 2. Hepp Z, Dodick DW, Varon SF, et al. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: A retrospective claims analysis. Cephalalgia. 2017;37:470-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: Results from the second international burden of migraine study (IBMS-II). Headache. 2013;53:644-655. [DOI] [PubMed] [Google Scholar]
- 4. Silberstein SD. Preventive migraine treatment. Continuum (Minneap Minn). 2015;21:973-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Migraine Foundation (AMF) . Preventive Treatments. Treasure Island; 2016. Available at: https://americanmigrainefoundation.org/resource-library/understanding-migrainepreventive-treatments/. Accessed April 2020. [Google Scholar]
- 6. Kumar A, Kadian R. Headache, migraine prophylaxis In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2018. [Google Scholar]
- 7. Bigal ME, Dodick DW, Krymchantowski AV, et al. TEV-48125 for the preventive treatment of chronic migraine: Efficacy at early time points. Neurology. 2016;87:41-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brandes J, Yeung PP, Aycardi E, et al. Early onset of action with fremanezumab versus placebo for the preventive treatment of episodic migraine (P4.107). Neurology. 2018;90:P4.107. [Google Scholar]
- 9. Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377:2113-2122. [DOI] [PubMed] [Google Scholar]
- 10. Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: A randomized clinical trial. JAMA. 2018;319:1999-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Camporeale A, Kudrow D, Sides R, et al. A phase 3, long-term, open-label safety study of Galcanezumab in patients with migraine. BMC Neurol. 2018;18:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Forderreuther S, Zhang Q, Stauffer VL, Aurora SK, Lainez MJA. Preventive effects of galcanezumab in adult patients with episodic or chronic migraine are persistent: Data from the phase 3, randomized, double‐blind, placebo-controlled EVOLVE‐1, EVOLVE‐2, and REGAIN studies. J Headache Pain. 2018;19:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Detke HC, Goadsby PJ, Wang S, Friedman DI, Selzler KJ, Aurora SK. Galcanezumab in chronic migraine: The randomized, double‐blind, placebo-controlled REGAIN study. Neurology. 2018;91:e2211-e2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baker B, Schaeffler B, Beliveau M, et al. Population pharmacokinetic and exposure-response analysis of eptinezumab in the treatment of episodic and chronic migraine. Pharmacol Res Perspect. 2020;8:e00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dodick DW, Goadsby PJ, Silberstein SD, et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: A randomised, double‐blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol. 2014;13:1100-1107. [DOI] [PubMed] [Google Scholar]
- 16. Dodick DW, Lipton RB, Silberstein S, et al. Eptinezumab for prevention of chronic migraine: A randomized phase 2b clinical trial. Cephalalgia. 2019;39:1075-1085. [DOI] [PubMed] [Google Scholar]
- 17. Ashina M, Saper J, Cady R, et al. Eptinezumab in episodic migraine: A randomized, double‐blind, placebo-controlled study (PROMISE7‐1). Cephalalgia. 2020;40:241-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lipton R, Goadsby PJ, Smith J, et al. PROMISE7‐2: Efficacy and safety of eptinezumab in patients with chronic migraine. Neurology. 2020;94:e1365-e1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dodick DW, Ashina M, Brandes JL, et al. ARISE: A phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38:1026-1037. [DOI] [PubMed] [Google Scholar]
- 20. Goadsby PJ, Reuter U, Hallstrom Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123-2132. [DOI] [PubMed] [Google Scholar]
- 21. Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim BK, Yang JY. Efficacy and safety of galcanezumab for the prevention of episodic migraine: Results of the EVOLVE‐2 phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38:1442-1454. [DOI] [PubMed] [Google Scholar]
- 22. Marcus R, Peritz E, Gabriel KR. On closed testing procedures with special reference to ordered analysis of variance. Biometrika. 1976;63:655-660. [Google Scholar]
- 23. Latham J, Karasek C, Ojala E, Allison D. Characterization of the binding of three anti-CGRP antibodies effective in preventing migraine: A comparative case study of ALD403, LY-2951742, TEV-48125. Cephalalgia. 2016;36:144. [Google Scholar]
- 24. Karasek C, Ojala E, Allison D, Latham J. Characterization of the intrinsic binding features of three anti-CGRP therapeutic antibodies effective in preventing migraine: A comparative pre-clinical case study of ALD403, LY-2951742, TEV-48125. Headache. 2016;56 :1-86. [Google Scholar]
- 25. Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, Ashman E. Evidence-based guideline update: Pharmacologic treatment for episodic migraine prevention in adults: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brandes JL, Saper JR, Diamond M, et al. Topiramate for migraine prevention: A randomized controlled trial. JAMA. 2004;291:965-973. [DOI] [PubMed] [Google Scholar]
- 27. Diener HC, Tfelt-Hansen P, Dahlof C, et al. Topiramate in migraine prophylaxis – Results from a placebo-controlled trial with propranolol as an active control. J Neurol. 2004;251:943-950. [DOI] [PubMed] [Google Scholar]
- 28. Silberstein SD, Neto W, Schmitt J, Jacobs D. Topiramate in migraine prevention: Results of a large controlled trial. Arch Neurol. 2004;61:490-495. [DOI] [PubMed] [Google Scholar]
- 29. Dodick DW, Turkel CC, DeGryse RE, et al. OnabotulinumtoxinA for treatment of chronic migraine: Pooled results from the double‐blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50:921-936. [DOI] [PubMed] [Google Scholar]
- 30. Reuter U, Goadsby PJ, Lanteri-Minet M, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: A randomised, double‐blind, placebo-controlled, phase 3b study. Lancet. 2018;392:2280-2287. [DOI] [PubMed] [Google Scholar]
- 31. Schwedt T, Reuter U, Tepper S, et al. Early onset of efficacy with erenumab in patients with episodic and chronic migraine. J Headache Pain. 2018;19:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goadsby PJ, Dodick DW, Martinez JM, et al. Onset of efficacy and duration of response of galcanezumab for the prevention of episodic migraine: A post-hoc analysis. J Neurol Neurosurg Psychiatry. 2019;90:939-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: A randomised, double‐blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15:382-390. [DOI] [PubMed] [Google Scholar]
- 34. Silberstein SD, Rapoport AM, Loupe PS, et al. The effect of beginning treatment with fremanezumab on headache and associated symptoms in the randomized phase 2 study of high frequency episodic migraine: Post-hoc analyses on the first 3 weeks of treatment. Headache. 2019;59:383-393. [DOI] [PubMed] [Google Scholar]
- 35. Detke HC, Millen BA, Zhang Q, et al. Rapid onset of effect of galcanezumab for the prevention of episodic migraine: Analysis of the EVOLVE studies. Headache. 2020;60:348-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hepp Z, Bloudek LM, Varon SF. Systematic review of migraine prophylaxis adherence and persistence. J Manag Care Spec Pharm. 2014;20:22-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data reported are part of an ongoing, global sponsor‐led clinical development and registration program. De‐identified participant data are not available for legal and ethical reasons.


