Figure 1. Bilateral optogenetic excitation of basal forebrain parvalbumin (BF-PV) neurons strongly reduced the latency to arousal from non-REM sleep.
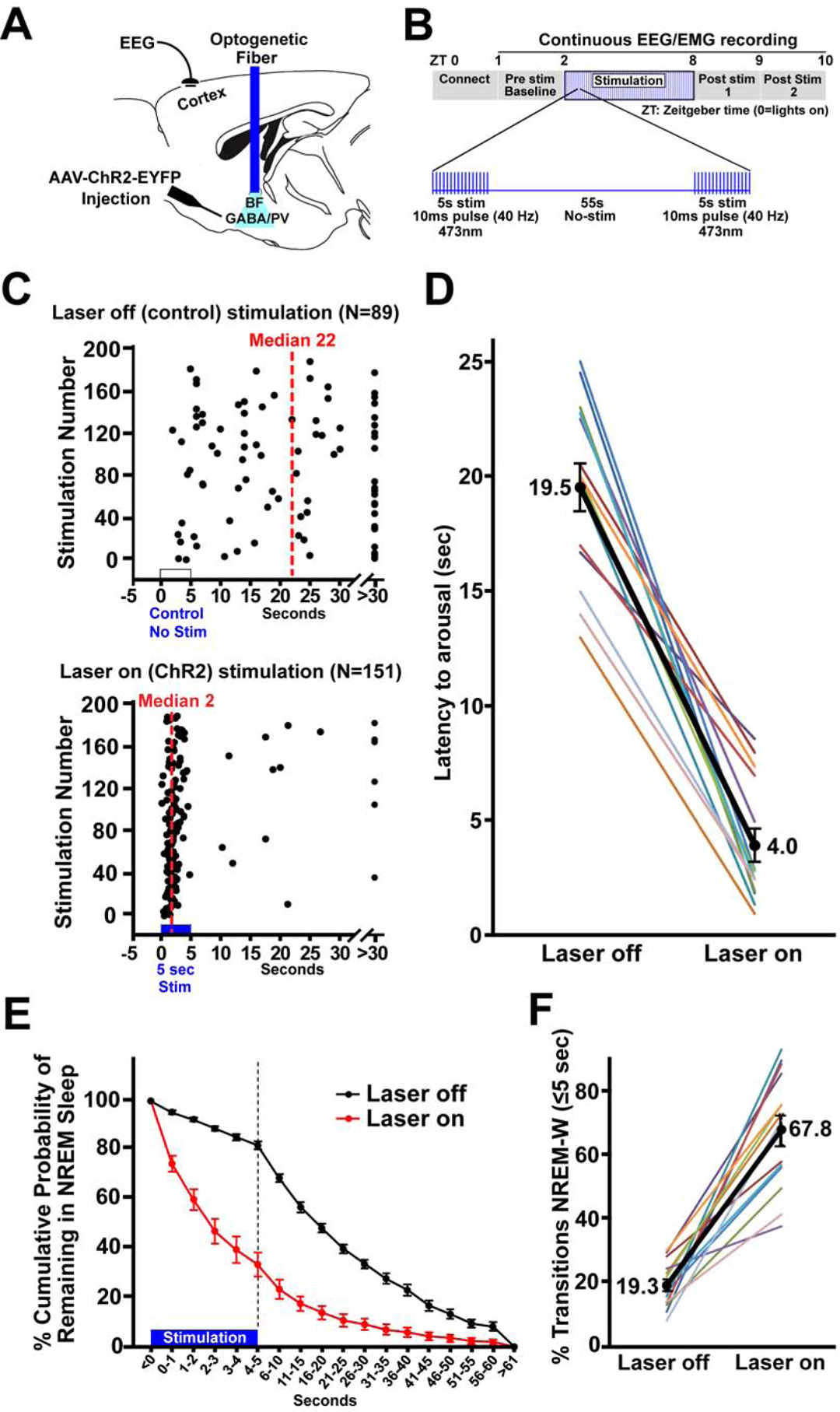
(A) Schematic of the experiment. Adeno-associated viral vectors with Cre-dependent expression of channelrhodopsin2-enhanced yellow fluorescent proteins (AAV-DIO-ChR2-EYFP) were bilaterally injected into the BF of PV-cre mice and blue laser light applied through an implanted optical fiber to excite BF-PV neurons. An electroencephalogram (EEG) electrode implanted above frontal cortex and an electromyogram electrode in the nuchal muscle (not shown) were used to assess behavioral state.
(B) Diagram illustrating the optogenetic stimulation protocol. 40 Hz stimuli with a 10 ms pulse duration were applied simultaneously to both sides of BF for 5 s, followed by 55 s of no stimulation, repeated for 6 hours (ZT2–8).
(C) Representative raster plots for one mouse illustrate a profound decrease in the latency for arousal from NREM sleep when stimulation was applied for 6 hours (ZT2–8).
(D) Group data for all mice show that optogenetic ChR2 stimulation produced a significant decrease (~4 fold) in median NREM-Wake latencies when compared to the laser off condition in the same mice (N=14).
(E) The cumulative probability distribution of NREM-to-wake transition latencies shows an increased probability for mice to be in wakefulness when given ChR2 stimulation compared to within animal, laser off control recordings. The probability of mice remaining in NREM sleep 5 s after the start of stimulation was 33.7%, in contrast to a probability of 82.0% at the same time point in control (laser off). The first 5 s are depicted per second, to demonstrate NREM probability distribution during ChR2 stimulation. 5 s bins are then depicted for the following 55 s.
(F) When considering all arousals, the majority (67.8%) of events occurred within 5 s when mice received bilateral stimulation of BF-PV neurons, whereas in controls only 19.3% of arousals occurred within 5 s; i.e the majority of NREM-to-Wake transitions (arousals) occurred within 5 seconds from the start of bilateral BF-PV optical stimulation.
