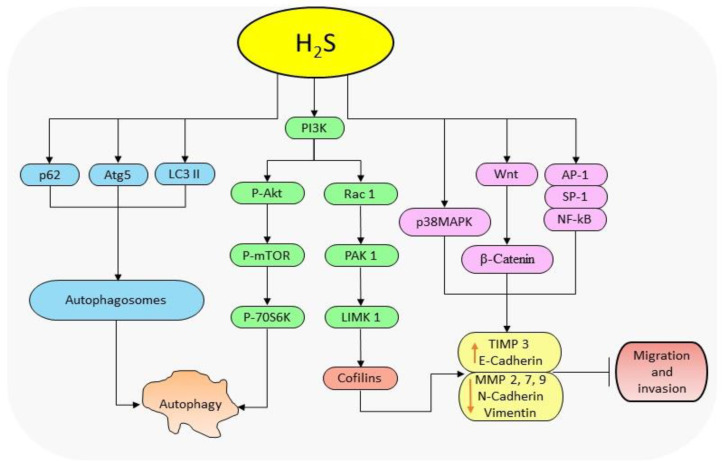
Figure 2.
The diagrammatic illustration of the mechanism by H2S donors in regulating autophagy and cell migration. H2S reduces the levels of p62 and elevates those of Atg5 and LC3-II to facilitate the formation of autophagosomes resulting in autophagy. Also, H2S suppresses the protein levels of p-PI3K, p-AKT and mTOR resulting in the downstream regulation of p-70S6K expressions and subsequently autophagy. By regulating PI3K, H2S also downregulates the expressions of Rac-1, PAK-1, and cofilin thereby reducing MMP-2, -7, -9, N-cadherin and vimentin levels, and increases TIMP 3 and E-cadherin levels resulting in the inhibition of cell migration. H2S further activates Wnt/β-catenin pathway to suppress migration activities, and inhibits NF-қB and MAPK pathways to reduce migration and invasion activities. (p62: nucleoporin 62; Atg5: autophagy-related protein 5; LC3 II: LC3-phosphatidylethanolamine conjugate; PI3K: phosphoinositide 3-kinase; AKT: protein Kinase B; mTOR: mammalian target of rapamycin; p-70S6K: ribosomal protein S6 kinase beta-1; Rac-1: Ras-related C3 botulinum toxin substrate 1; PAK-1: p21-activated kinase 1; MMPs: matrix metalloproteinases; TIMP 3: tissue inhibitor of metalloproteinase-3; MAPK: mitogen-activated protein kinase; NF-кB: nuclear factor-kappa B).