Figure 1.
Generation of Intra-embryonic Hematopoietic Progenitors from hPSCs Using a Spin-EB Method
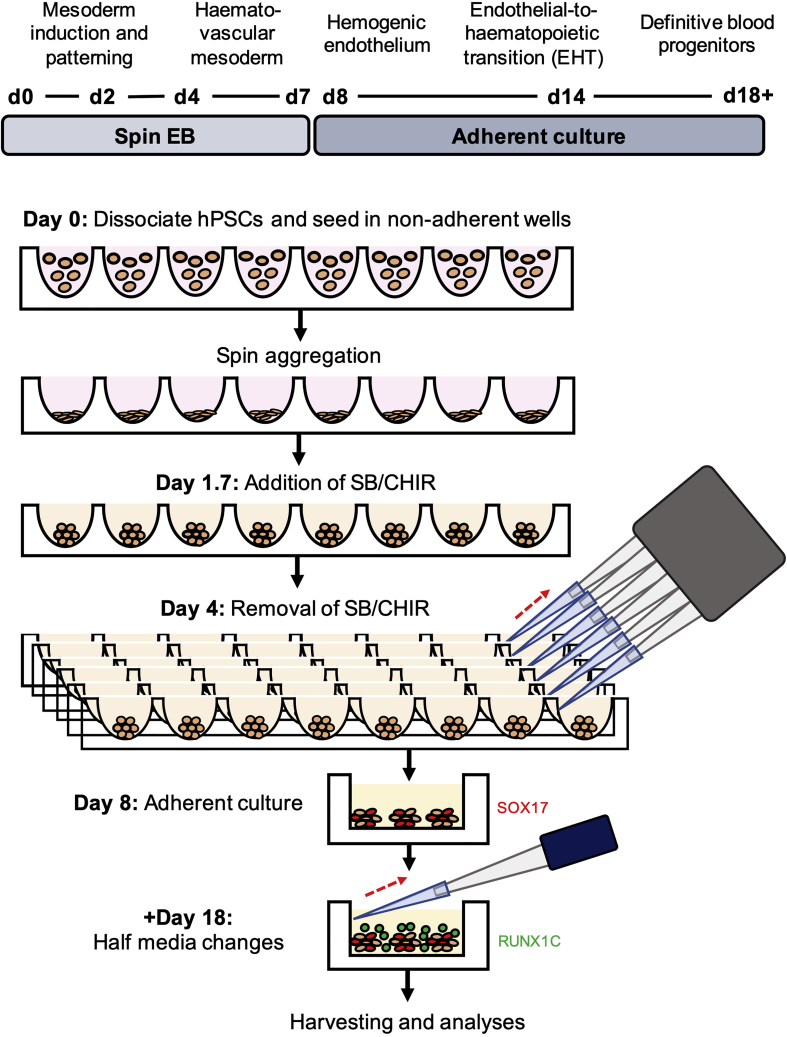
The timeline of the protocol is shown at the top of the figure. In order to set up the spin-EB culture, single-cell dissociated hPSCs are plated in non-adherent round-bottom 96-well plates and are aggregated at the bottom of the wells by centrifugation, yielding 60 EBs/plate. EBs are cultured in Stage 1 medium for 4 days. At day 1.7, EBs are treated with an Activin inhibitor (SB) and WNT-agonist (CHIR) to pattern the mesoderm toward the intra-embryonic, HOXA-positive hematopoietic program, also inhibiting the generation of extra-embryonic blood progenitors. At day 4, medium containing SB/CHIR is removed using a multichannel pipette, placed in an angle to not disturb the non-adherent EBs present at the bottom of the wells. EBs are subsequently cultured in Stage 2 growth factors that promote endothelial development until day 8, when EBs are transferred to adherent wells in Stage 3 media to allow the outgrowth of stromal and endothelial cells. EB cultures generate CD34+ RUNC1C+ blood progenitors that arise from cell clusters located within arterial structures of SOX17+ hemogenic endothelium. Hematopoietic progenitors detach from the endothelium and continue maturing in suspension. To avoid removal of blood progenitors in suspension, half medium changes are performed from day 14, by tilting the pipette and removing only the upper 2 mL of medium and then adding 2 mL of fresh medium. Blood progenitors and EBs can be harvested at different stages of the differentiation and subjected to a range of analyses, including gene and protein expression, chromatin accessibility and immunoprecipitation, and growth and differentiation assays.