Summary
Chronic cranial window surgery is a critical procedure for in vivo imaging in neuroscience. Here, we describe our surgical protocol with several subtle improvements that increase the success rate significantly. The window allows high-quality imaging in head-fixed behaving mice within the first week after the surgical procedure and remains clear for months. We used this procedure to prepare mice for intrinsic signal imaging and two-photon imaging of layer 6 neurons in visual cortex.
For complete details on the use and execution of this protocol, please refer to Augustinaite and Kuhn (2020).
Subject Areas: Microscopy, Model Organisms, Neuroscience
Graphical Abstract
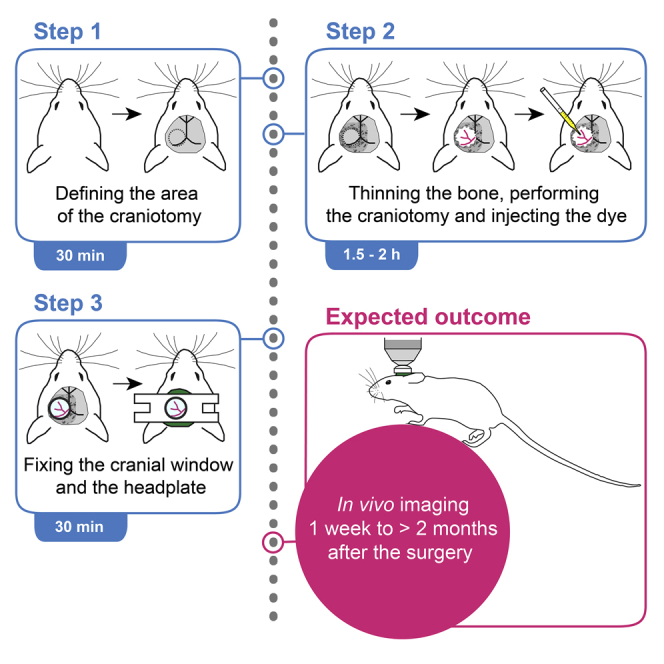
Highlights
-
•
Long-term, high-quality chronic cranial window for imaging applications
-
•
Instructions for craniotomy and mounting of a cranial window with headplate
-
•
Instructions for tracer, dye, and/or virus injection during the surgery
-
•
Specific instructions for surgeries over primary visual cortex (V1) in mice
Chronic cranial window surgery is a critical procedure for in vivo imaging in neuroscience. Here, we describe our surgical protocol with several subtle improvements that increase the success rate significantly. The window allows high-quality imaging in head-fixed behaving mice within the first week after the surgical procedure and remains clear for months. We used this procedure to prepare mice for intrinsic signal imaging and two-photon imaging of layer 6 neurons in visual cortex.
Before You Begin
Here we describe materials and methods for a mouse chronic cranial window (Holtmaat et al., 2012) surgery over the primary visual cortex (V1) combined with fluorescent tracer and viral vector injection to the dorsal lateral geniculate nucleus (dLGN) to label thalamocortical axons and layer 6 corticothalamic neurons (Augustinaite and Kuhn, 2020). The stereotaxic coordinates for a different window location or injection site can be obtained from any mouse brain atlas (e.g., Paxinos and Franklin, 2013).
CRITICAL: All animal experiments must be performed in accordance with guidelines approved by the Institutional Animal Care and Use Committee (IACUC). The usage of narcotic/psychotropic substances must be coordinated with local legislation authorities.
Prepare Pipettes for Injections
Timing: 10 min per pipette
-
1.
Pull a thick wall quartz pipette (1 mm outer diameter, 0.3 mm inner diameter; Sutter Instrument) using a laser puller (P-2000, Sutter Instrument).
-
2.
Bevel the pipette at 35° angle to a sharp needle-like tip with about 10–15 μm opening diameter (short axis of the elliptic opening; BV-10, Sutter Instrument).
CRITICAL: The tapped-out shank of the pipette should be about 2–4 mm long and narrow (Figures 1A and 1B) to minimize tissue damage during injection. At the same time, it should remain rigid for penetration of the dura. Therefore, we prefer thick-walled quartz pipettes over borosilicate glass pipettes.
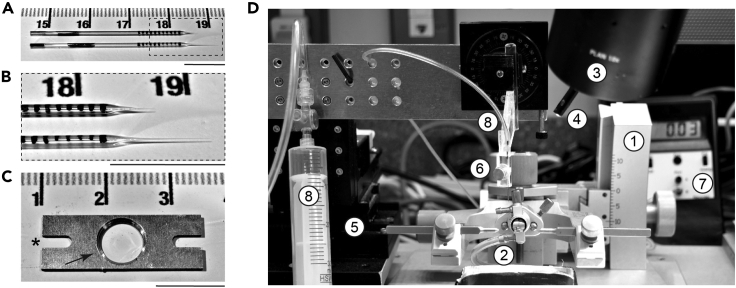
Figure 1.
Preparation for the Chronic Cranial Window Surgery and Injection of Beads, Viral Vectors, Dyes, Drugs, or Other Solution
(A and B) (A) Quartz pipettes for injections; (B) magnified tips.
(C) Headplate for fixation of a mouse on an imaging stage. ∗ indicates a slit for fixation screw, arrow points at the cut edge of the central opening.
(D) Surgery setup consisting of a (1) stereotaxic frame with (2) nose cone connected to an anesthesia apparatus, (3) stereo microscope, (4) LED light source, (5) micromanipulator, and (6) pipette holder connected to (7) a manometer by silicone tubing for manual pressure application with (8) a syringe. Scale bar in (A)–(C), 1 cm.
-
3.
To judge the injection volume of the pipette, draw tick marks, 1 mm apart, with a fine-tip water-resistant marker. When using a pipette with 0.3 mm inner diameter and taking up a liquid, the rise of the meniscus inside the pipette by 1 mm corresponds to a volume of 70 nL (Figures 1A and 1B).
Prepare Headplates for the Fixation of the Mouse under the Two-Photon Microscope
Timing: 10 min per headplate
-
4.
Cut the headplate (24 × 8 mm, 6 mm central opening diameter, two 4 × 2 mm slits for fixation screws; Figure 1C, asterisk) out of a hard aluminum alloy plate (1 mm thick, A2017) using machine shop equipment.
-
5.
Cut the edge of the central opening on one side of the headplate (Figure 1C, arrow).
CRITICAL: The headplate design must fit to the headplate holder design on the imaging stage.
Prepare Surgical Tools and Equipment for Cranial Window Surgery
Timing: 30 min
-
6.
Sterilize surgical tools: scalpel, small scissors, 2× tweezers and 2× carbide drill bits (0.5 mm and 1.0–1.2 mm) in the glass-bead sterilizer (15 s at 260° C, Germinator 500, Cellpoint Scientific).
-
7.
Prepare the surgery setup (Figure 1D) consisting of a stereotaxic frame (1; Model 1900, KOPF Instruments) with nose cone connected to an anesthesia apparatus (2; MK-AT210, Muromachi Kikai), stereo microscope (3; M80, Leica microsystems), LED light source (4; KL 1600 LED, Schott), micromanipulator (5; MP-285, Sutter Instrument), and a pipette holder (6; electrode holder H-2, Narishige) connected to a manometer (7; SYS-PM015D, W.P.I.) by silicone tubing for manual pressure application with a syringe (8).
Alternatives: Commercially available pneumatic or hydraulic microinjectors can be used instead of the manual pressure application system.
-
8.
Turn on the LED light, micromanipulator, dental drill (EXL-M40, Osada), and anesthesia apparatus. Check that sufficient isoflurane is in the anesthesia apparatus.
-
9.
Mentally go over the surgery and check that all necessary drugs, tools, and consumables are at hand.
-
10.
Sterilize the stereotactic frame and tool table surface with alcohol (70% ethanol).
-
11.
Cover the tool table with a sterile sheet of paper towel and place the sterilized tools and other items on it.
-
12.
Prepare a fresh cage for the mouse.
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| AAV1.hSyn.TurboRFP.WPRE.rBG | Addgene | 105552-AAV1 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| IsoSol Isoflurane USP | Vedco | NDC 50989-150-15 |
| Medetomidine (Domitor, 10 mL) | Nippon Zenyaku Kogyo | N/A |
| Atipamezole hydrochloride | Nippon Zenyaku Kogyo | N/A |
| Midazolam (Dormicum injection, 10 mL) | Maruishi Pharmaceutical | N-A |
| Butorphanol (Vetorphale, 5 mg) | Meiji Seika Pharma | N/A |
| Carprofen (Rimadyl injection) | Zoetis Japan | N/A |
| Dexamethasone sodium phosphate | Alfa Aesar | AAJ6408303 |
| Buprenorphine (Lepetan injection, 0.3 mg) | Otsuka Pharmaceutical | N/A |
| Lidocaine 2% | Nagase Medicals | N/A |
| Iodine tincture 3 g/100 mL | Kenei Pharmaceutical | N/A |
| Ophthalmic ointment Mycochlorin | Sato Pharmaceutical | N/A |
| Phosphate-buffered saline (PBS, pH 7.4) | Thermo Fisher Scientific | 10010031 |
| Critical Commercial Assays | ||
| FluoSpheres, Fluorescent Color Kit, carboxylate | Molecular Probes | F10720 |
| Other | ||
| DietGel 76a | Clearh2o | https://www.clearh2o.com |
| Dental adhesive resin cement Super-Bond C&B | Sun Medical | https://www.sunmedical.co.jp/english/index.html |
| Quartz glass OD, 1 mm; ID, 0.3 mm, 7.5 cm length | Sutter Instrument | Q 100-30-7.5 |
| Cover slips, MINI, 5 mm DIA. | W.P.I. | 502040 |
| Micropipette puller P-2000 | Sutter Instrument | https://www.sutter.com/MICROPIPETTE/p-2000.html |
| Microelectrode beveler BV-10 | Sutter Instrument | https://www.sutter.com/MICROPIPETTE/bv-10.html |
| Germinator 500 glass-bead sterilizer | Cellpoint Scientific | http://www.cellpointscientific.com/Products/Germinator-Dry-Glass-Bead-Sterilizer/GERMINATOR-500-GLASS-BEAD-STERILIZER_2 |
| Model 1900 Stereotaxic alignment system | KOPF Instruments | https://kopfinstruments.com/product/model-1900/ |
| Electrode holder H-2 | Narishige | https://www.tritechresearch.com/H-2.html |
| Anesthetic vaporizer MK-AT210 | Muromachi Kikai | N/A |
| Routine stereo microscope M80 | Leica microsystems | https://www.leica-microsystems.com/products/stereo-microscopes-macroscopes/p/leica-m80/ |
| Light source for fiber optics Schott KL 1600 LED | Schott | https://www.schott.com/lightingimaging/english/microscopy/products/kl/1600led.html |
| MP-285 Micromanipulator system | Sutter Instrument | https://www.sutter.com/MICROMANIPULATION/mp285.html |
| Pressure manometer SYS-PM015D | W.P.I. | https://www.wpiinc.com/var-3529-pressure-manometer |
| Dental laboratory handpiece and Motor system EXL-M40 | Osada | https://www.osadausa.com/exlm40.html |
| Hair clipper Wahl KM2 Model 9757-200 | Wahl | https://www.wahlanimal.com/product/km2-clipper-kit/ |
| Ultrasonic processor Vibra-Cell VCX 500 | Sonics | https://www.sonics.com/liquid-processing/products/vibra-cell-processors/ |
| Surgical tools | Fine Science Instruments, W.P.I. |
https://www.finescience.com/en-US/ https://www.wpiinc.com/surgical-instruments |
| Sterile sponge points Sugi | Questalpha | 31602 |
| Gel-foam sterile sponge No.12 | Pfizer | 09-0315-08 |
| Wrapped cotton swabs TX705 | Texwipe | https://www.texwipe.com/swabs |
| Industrial Q-tips AP-5 | As One | 1-8584-05 |
| Experimental Models: Organisms/Strains | ||
| Ntsr1-Cre mouse line GN220Gsat strain B6.FVB(Cg)-Tg(Ntsr1-Cre) | Mutant Mouse Regional Resource Center (MMRRC) | 030648-UCD |
| C57BL6J mouse | Japan Clea | N/A |
Materials and Equipment
All materials, equipment, and their sources are listed in the text and in the Key Resources Table.
Alternatives: For alternative materials, equipment, and their sources consult with local medical, pharmaceutical, and laboratory equipment suppliers.
Step-By-Step Method Details
Preparation of the Mouse for Chronic Cranial Window Surgery
Timing: 15 min
Prepare the 2–3 months old mouse for the surgery, before fixing it to the stereotaxic frame.
CRITICAL: The procedure and drugs should be adapted according to your IACUC approved protocol.
-
1.
Weigh the animal.
-
2.Induce anesthesia.
-
a.Gently transfer the mouse into an anesthesia induction chamber and induce deep anesthesia with 3% isoflurane.Note: Anesthesia should induce within 1–1.5 min.Alternatives: Researchers experienced with mouse handling can skip this step.
-
b.Inject main anesthetics; here, mixture of Medetomidine (dissolved in 0.9% saline; 0.3 μg/g body weight), Midazolam (dissolved in 0.9% saline; 4 μg/g), and Butorphanol (dissolved in 0.9% saline; 5 μg/g intraperitoneal [i.p.]) before isoflurane wears off.
-
a.
Note: Anesthesia will be induced within 3–5 min. Therefore, leave the mouse in its cage for 5 min for a smooth transition to deep anesthesia.
Alternatives: Researchers using isoflurane as the main anesthesia should fix the mouse in the stereotaxic frame (here, step 8) and proceed with step 4.
-
3.
Transfer the mouse onto a sterile paper towel.
-
4.
Inject immunosuppressants; here, Carprofen (dissolved in 0.9% saline; 7.5 μg/g body weight, i.p.) and Dexamethasone sodium phosphate (dissolved in 0.9% saline; 2 μg/g body weight, into the quadriceps muscles, 1 μg/g each).
CRITICAL: Without immunosuppressants, a strong background autofluorescence in the dura and brain tissue will be caused by a strong immune system response to the introduced foreign bodies (for example, glass coverslip or fluorescent tracer) and healing. This will substantially reduce the imaging quality. Troubleshooting Problem 1.
-
5.
Apply ophthalmic ointment to the eyes for physical protection and to prevent dehydration using small cotton-tips.
CRITICAL: Extensively applied highly viscous ophthalmic ointment (for example, Mycochlorin) will protect the eyes from cut hair as well as accidental contact with hair-removal cream, iodine, or ethanol (steps 6 and 8).
-
6.Remove the scalp hair.
-
a.Shave the head with a veterinary hair clipper (KM2, Model 9757-200, Wahl).Note: Use a clipper blade specifically for small animals with fine hair.
-
b.Apply hair-removal cream over the shaved scalp and clean it off (here, after 5 min) using small cotton-tips.
 CRITICAL: Keep hair-removal cream away from the eyes of the mouse.
CRITICAL: Keep hair-removal cream away from the eyes of the mouse. CRITICAL: Check instruction of the cream. Also note, that the application time can be less than indicated and therefore should be adjusted. Too long application might irritate or damage the skin.Note: Use hair-removal cream for sensitive skin.Note: Avoid excessive usage of hair-removal cream.
CRITICAL: Check instruction of the cream. Also note, that the application time can be less than indicated and therefore should be adjusted. Too long application might irritate or damage the skin.Note: Use hair-removal cream for sensitive skin.Note: Avoid excessive usage of hair-removal cream. -
c.Optional: The iodine or 70% ethanol can be applied for immediate disinfection and wash-off residual hair-removal cream (see also step 8)
-
a.
Preparation for the Craniotomy
Timing: 40–60 min
CRITICAL: The craniotomy is the most critical part of the cranial window surgery. Correctly performed, it will lead to a chronic cranial window which enables long-term, high-quality imaging (see Expected Outcomes) while imperfections lead to dissatisfactory imaging quality or even animal death (see Troubleshooting Problems 1–4).
-
7.
Fixate the head in a stereotaxic frame using blunt ear bars.
-
8.
Wipe the scalp with cotton swabs soaked in iodine.
CRITICAL: Keep iodine away from the eyes of the mouse.
Note: Avoid excessive usage of the iodine.
-
9.
Apply Lidocaine topically for local anesthesia using a cotton swab. Wait 3–5 min for induction of local anesthesia (Figure 2A).
-
10.Remove the scalp skin (Figure 2B).
-
a.Open the skin above the skull making one midline stroke with a scalpel. The cut should start just behind the eyes (2–3 mm anterior to bregma) and end at the posterior end of the skull (above the neck).Note: The length and position of the incision should be adapted for craniotomies at other locations.
-
b.Cut away the scalp skin on both sides of the incision.Note: Only skin without hair should be removed. Otherwise cut hair will irritate the wound.
-
c.Push remaining skin to the side using Lidocaine-soaked cotton-tips.
-
d.Scrape connective tissue and muscles away from the lateral and posterior bone ridges using tweezers.
-
a.
CRITICAL: The large open-bone area (Figure 2B) is necessary for a sufficient bone thinning around the craniotomy as well as secure fixation of the headplate using dental acrylic (see steps 12 and 22–25; Troubleshooting Problem 4).
-
11.Define the position of the craniotomy (Figure 2C).
-
a.Level the skull along the midline axis at the suture intersection points bregma and lambda using a reference pipette (a relatively short-shank injection pipette as in Figures 1A and 1B; the tip of this pipette can be colored with a water-resistant marker for better visibility) orthogonally fixed on a micromanipulator (Figures 1D and 2C). Also make sure that the skull is not tilted in the horizontal plane if your stereotactic apparatus allows to tilt the ear bars.
-
b.Lower the pipette onto bregma. Zero the coordinates.
-
c.Locate the center of the future cranial window over V1 with the reference pipette: 3.5 mm posterior and 2.4 mm lateral of bregma. Go up with the pipette and mark the location below the pipette with a fine-tip water-resistant marker.
 CRITICAL: All coordinates should be adapted for different target regions.
CRITICAL: All coordinates should be adapted for different target regions. -
d.Mark anterior, lateral, and medial points 1.5 mm and posterior point about 3 mm from the center.
 CRITICAL: Here, the posterior mark is behind the transverse sinus (asterisk in Figure 3). Otherwise, the drilling around the future craniotomy area or bone breaking during the craniotomy (see steps 12 and 14) would likely damage this large vein, which would lead to strong bleeding and ultimately death of the animal. Troubleshooting Problem 2 and Problem 3.
CRITICAL: Here, the posterior mark is behind the transverse sinus (asterisk in Figure 3). Otherwise, the drilling around the future craniotomy area or bone breaking during the craniotomy (see steps 12 and 14) would likely damage this large vein, which would lead to strong bleeding and ultimately death of the animal. Troubleshooting Problem 2 and Problem 3. -
e.Move the pipette out of the workspace without changing the bregma zero coordinates.Note: Keeping the bregma zero position, especially the ventral zero position, is essential if the bone under bregma will be thinned (see step 12).
- f.
-
a.
Note: Do not drill deep. This groove is only for marking the future craniotomy.
-
12.
Thin the bone around the engraving with a 1- or 1.2-mm diameter drill bit using a drill speed of 3×104 rpm. The bone should be thinned most around the engraving while bone further away should be thinned less (Figures 2E and 3B). Stop thinning when tiny cracks appear around the engraving or when the bone inside the engraving can be easily pushed when applying gentle force with the tweezers.
CRITICAL: Here and anytime when drilling: Do not break through the bone. Since the dura in mice is very thin, the drill bit will sink into the brain immediately, causing strong bleeding and big damage to the brain. The mouse should be sacrificed in such a case. Troubleshooting Problem 2 and Problem 3.
CRITICAL: Drill carefully, with smooth motion, keeping the drill at a shallow angle and applying minimal pressure with the drill bit. Drilling for too long at the same position will cause over-heating and damage the dura, blood vessels, and the brain. Such damage can be noticed as bruises or bleeding below the bone in the thinned-skull area. Periodically cool and clean the bone with saline and gentle spray with compressed air. Reckless drilling will cause long-lasting inflammation leading to high autofluorescence in the brain tissue, neuronal death, and, accordingly, strongly reduced two-photon image quality. Troubleshooting Problem 1.
Note: In our experience, only hands should move, while arms should remain immobile and pressed against the stereotactic frame for better control of the drill.
Note: A small cotton-tip or a flat-tip forceps in the contralateral hand, slightly pressing the skull also helps for control and stability.
Note: The area of thinned bone and the thickness of the remaining bone depends on the age of the animal (older mice have thicker skulls) and the size as well as the position of the headplate. Keep in mind that larger area ensures strong fixation of the headplate (see step 22–25, Figures 2K and 2L). Troubleshooting Problem 4. Also, the headplate should be as close to the dura as possible to prevent the steric problems with large, short working distance imaging objectives.
Note: The cranial window can be glued directly on the dura (see step 21). This and the very thin bone at the edge of the craniotomy (Figures 3B–3D) prevent the re-growth of the bone under the glass. Troubleshooting Problem 1.
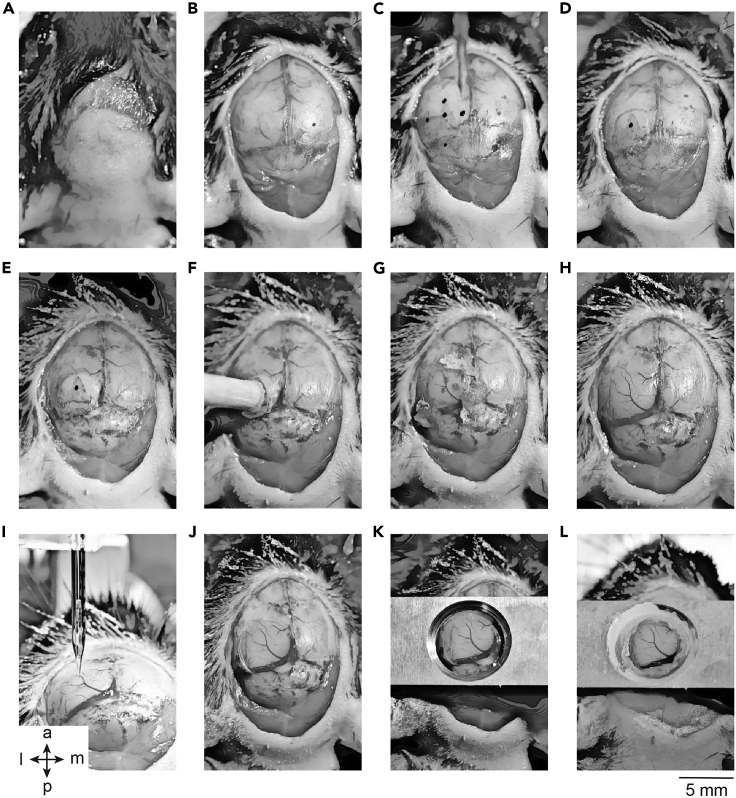
Figure 2.
Major Steps of the Chronic Cranial Window Surgery
(A) Clean the scalp.
(B) Remove the scalp skin and clean the skull.
(C) Define the position of the craniotomy.
(D) Engrave around the area of the craniotomy.
(E) Thin the bone around the craniotomy area.
(F) Glue the wooden stick for lifting the craniotomy bone.
(G) Clean the exposed dura.
(H) Clean craniotomy area prepared for the injection.
(I) Injection.
(J) Glued chronic cranial window.
(K) Temporally fixed headplate.
(L) Headplate glued with dental acrylic. The orientation: a, anterior; p, posterior; l, lateral; m, medial.
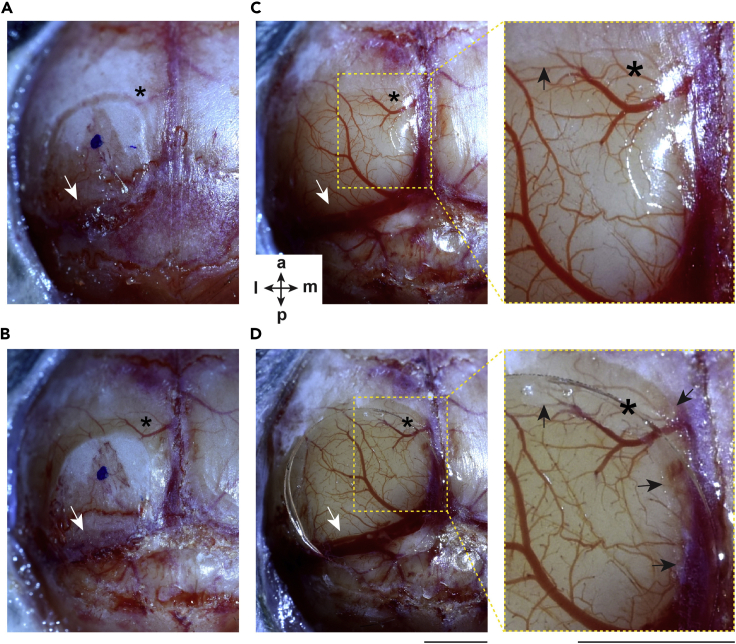
Figure 3.
Key Aspects of the Chronic Cranial Window Surgery
(A and B) (A) Before and (B) after the thinning of the skull bone. Notice the different thickness of the bone closer and further away from the engraved area of the craniotomy.
(C) The craniotomy after cleaning; enlarged image (right). Black arrow indicates the edge of the craniotomy.
(D) Glued chronic cranial window; enlarged image (right). Black arrows indicate the glue on the bone/dura below the coverslip. Notice that the achieved craniotomy area is larger than defined initially (see asterisks as reference points). White arrow points at the transverse sinus. Scale bars, 2 mm. The orientation: a, anterior; p, posterior; l, lateral; m, medial.
The Craniotomy
Timing: 15–20 min
With minor modifications we are following a procedure described earlier (Roome and Kuhn, 2014).
-
13.
Glue a wooden stick (about 3 mm diameter, as, for example, the wooden part of a cotton swab) vertically to the center of the craniotomy bone with gel superglue. Press it slightly against the bone for 10–15 s and let it set for 5 min (Figure 2F).
Note: Gel or high-viscosity superglue should be used to avoid leakage onto the thinned bone area and to fill the space between the bone and the stick for maximal bonding.
Note: Use a stick with a diameter slightly smaller than the craniotomy bone. The glue will spread over the thinned bone area if using a stick with larger diameter, although a too thin stick might get de-attached from the bone when pulled.
-
14.
Gently lift and slightly tilt the stick backward to separate the attached bone patch from the dura while pressing the anterior part of the skull downwards with a cotton swab or the flat side of the tweezers. The resulting craniotomy should have a diameter of 4–5 mm.
CRITICAL: If the bone does not break, but some cracks are visible, additionally use fine-tip tweezers when lifting the stick. Pulling the bone up will minimize the risk of dura damage with forceps and help to cut-open the skull. If no cracks are visible, or appear when lifting, remove the stick by scraping the glue from the bone using tweezers and proceed with more thinning of the bone around the engraving.
CRITICAL: Lifting the bone might damage blood vessels and cause bleeding. Be prepared to use a sterile sponge (Sugi, Questalpha) and gel-foam sponge (No. 12, Pfizer) moisturized with saline or Carprofen.
CRITICAL: Damage to the large blood vessels or sinuses will cause strong bleeding which can lead to death during or up to 48 h after the surgery. Troubleshooting Problem 2 and Problem 3.
-
15.
Clean the dura with gel-foam sponge (No. 12, Pfizer) moisturized with saline or Carprofen (Figure 2G). No bleeding should be observed after adding a drop of saline on the dry dura (Figures 2H and 3C).
CRITICAL: Gluing the window before the damaged blood vessels are cured will cause bleeding during the recovery period. Residual blood below the cranial window will seriously affect the quality of two-photon imaging. Troubleshooting Problem 1.
Retrograde Tracer Injection
Timing: 15 min
The following steps provide instructions on how to inject a solution into a specific brain area. Here, we explain how to inject retrogradely transported fluorescent beads (FluoSpheres, Molecular Probes) and viral vector (AAV1.hSyn.TurboRFP.WPRE.rBG, Addgene) into dorsal lateral geniculate nucleus (dLGN).
Note: These steps can be omitted if the solution of interest (for example, a viral vector, dye, drugs, or beads) will be injected through an access port of the chronic cranial window at a later time point (Roome and Kuhn, 2014, Roome and Kuhn, 2018, Augustinaite and Kuhn, 2020).
-
16.Installing the injection pipette.
-
a.Return the reference pipette (step 11e) to the zeroed bregma coordinates. If the bone was thinned over the bregma point, record the difference in ventral direction between 0 and the bone surface by lowering the pipette tip onto the bone (you will need this offset in step 16e).
-
b.Remove the reference pipette.
-
c.Connect the silicone tubing to the end of the injection pipette and fix it in the holder.Note: Select a pipette with short shaft if injecting into the upper cortex. If the injection is aiming for deep cortex (layers 5 or 6) or sub-cortical areas, use a pipette with long shaft to minimize tissue damage during insertion (Figures 1A and 1B).
-
d.Carefully lower the pipette tip onto the bone of the bregma. Zero the coordinates.
-
e.Lift the pipette up the recorded distance from 0 in step 16a. Zero the coordinates.
-
a.
CRITICAL: Wrong zeroed bregma coordinates will lead to an inaccurate injection location and to failure of the labeling. Troubleshooting Problem 5.
-
17.Tip-fill the pipette with a tracer containing solution.
-
a.Cut a 5×10 mm piece of parafilm with the protective wax paper attached.
-
b.Mix a 2 μL drop of tracer solution (mixed 1:1 with PBS and sonicated for 5–10 s (ultrasonic processor VCX 500, Sonics) immediately before the filling) with 2 μL drop of AAV solution on the parafilm.
-
c.Remove the protective wax paper and place the parafilm on the ear-bar, within the working distance of the micromanipulator.
-
d.Apply weak positive pressure (10–14 hPa) and lower the pipette tip into the drop under visual guidance.Note: Since the tracer solution has a high viscosity, such weak positive pressure is enough to keep the tip clean while in the air and at the droplet surface, but without making air bubbles when in the droplet.
-
e.Tip-fill the pipette with up to 300 nL of solution using negative pressure (typically −150 to −200 hPa). The volume is judged by the tick marks on the pipette.
 CRITICAL: Replace the pipette in case of clogging (remove the clogged pipette and go back to step 16c). Try a larger tip diameter pipette and/or additional dilution with PBS.
CRITICAL: Replace the pipette in case of clogging (remove the clogged pipette and go back to step 16c). Try a larger tip diameter pipette and/or additional dilution with PBS. -
f.Apply weak positive pressure (10–14 hPa) and lift the pipette up. Maintain this pressure until the injection of the solution into the brain (step 18c).
 CRITICAL: The positive pressure is kept to counteract the capillary effects. It prevents air or cerebrospinal fluid (step 18a, b) to tip-fill the pipette. If air is taken up, it will be injected into the brain; if cerebrospinal fluid is taken up, the tracer solution will be diluted.On the other hand, if too high positive pressure is applied, the tracer solution will be ejected, emptying the pipette before entering the brain, or causing strong labeling along the pipette tract in the brain. Therefore, the pressure should be adjusted depending on the viscosity of the solution as well as inner diameter of the injection pipette. Troubleshooting Problem 5.
CRITICAL: The positive pressure is kept to counteract the capillary effects. It prevents air or cerebrospinal fluid (step 18a, b) to tip-fill the pipette. If air is taken up, it will be injected into the brain; if cerebrospinal fluid is taken up, the tracer solution will be diluted.On the other hand, if too high positive pressure is applied, the tracer solution will be ejected, emptying the pipette before entering the brain, or causing strong labeling along the pipette tract in the brain. Therefore, the pressure should be adjusted depending on the viscosity of the solution as well as inner diameter of the injection pipette. Troubleshooting Problem 5. -
g.Wipe the pipette tip with a PBS-soaked sterile sponge (Sugi, Questalpha).
-
a.
Note: Fluorescent particles/viral vectors outside the glass wall might label neurons/tissue along the insertion track and thus compromise the labeling and/or the imaging quality. Troubleshooting Problem 5.
-
18.Inject the tracer (Figure 2I).
-
a.Locate the pipette above the target and smoothly lower it into the brain using bregma as the origin of the coordinate system (2.45 mm posterior, 2.4 mm lateral, 2.9 mm ventral for dLGN).
-
b.Wait for 3–5 min at the target location allowing the tissue to seal around the pipette wall before the injection.
 CRITICAL: Immediate injection will cause the spread of the tracer solution up along the pipette instead of into the tissue at the injection site. Troubleshooting Problem 5.
CRITICAL: Immediate injection will cause the spread of the tracer solution up along the pipette instead of into the tissue at the injection site. Troubleshooting Problem 5. -
c.Under visual observation of the meniscus in the pipette, if possible, with the surgery microscope, pressure inject (typically 35–70 hPa) about 150–200 nL of solution within 10–15 min.
-
d.Reduce the positive pressure to initial 10–14 hPa and wait for 10 min to allow the injected solution to spread into the tissue.
 CRITICAL: Visual observation allows the accurate control of the injection speed and the amount. Slow injection (50 nL/3–5 min) and a waiting period before and after the injection, allows the solution to spread locally at the injection site instead of going up along the outer walls of the pipette during injection or follow the pipette track during retraction. Troubleshooting Problem 5.
CRITICAL: Visual observation allows the accurate control of the injection speed and the amount. Slow injection (50 nL/3–5 min) and a waiting period before and after the injection, allows the solution to spread locally at the injection site instead of going up along the outer walls of the pipette during injection or follow the pipette track during retraction. Troubleshooting Problem 5. -
e.Slowly and smoothly retract the pipette.
-
a.
Fixing the Cranial Window
Timing: 10 min
The following steps provide instructions on how to cover the craniotomy with the cranial window (round glass coverslip MINI, 0.17 mm thick, 5 mm diameter; W.P.I.; Figures 2J and 3D).
Optional: Due to the curvature of the skull, the lateral side of the craniotomy is lower than the medial one (Figure 2I). Tilt the head of the mouse by 5°–10° in the horizontal plane to level the edges of the craniotomy on the latero-medial axis (compare Figure 3C with 3D) if your stereotactic apparatus allows to tilt the ear bars.
Note: The leveling makes it easier to glue the coverslip and, afterwards, to mount the headplate parallel to this coverslip.
-
19.
Before covering the craniotomy, clean the coverslip with alcohol and compressed air.
-
20.
Place the coverslip on the dura and slightly press it with a small cotton-tip so that the glass attaches to the dura on a large area of the craniotomy.
CRITICAL: If the brain surface appears concave (because of low blood pressure due to blood loss or deep anesthesia), add a drop of saline onto the center of the craniotomy. The water will prevent the glue from filling the space below the coverslip. Troubleshooting Problem 1.
CRITICAL: Do not proceed if bleeding starts. Remove the coverslip and clean the dura as explained in step 15. Troubleshooting Problem 1.
CRITICAL: The dura should be kept intact throughout the surgery. Attempts of dura removal can lead to bleeding and brain damage (Troubleshooting Problem 1 and Problem 2). In mice, the dura is transparent and thin, and therefore allows two-photon imaging even at a depth of 600–800 μm (Augustinaite and Kuhn, 2020). If, however, the dura must be removed, special care must be taken to prevent damage of the brain, especially of cortical layer 1. For example, the dura can be separated from the pia mater using hydroperoxide (Dalphin et al., 2020) before opening it with fine-tip forceps.
-
21.
While pressing in the center with a small cotton-tip, glue the coverslip by applying small drops of a liquid superglue to the edges of the glass (Figures 2J and 3D). To do this, put a drop of regular superglue on a piece of parafilm and pick up droplets with a narrow but flat end wooden stick (for example, broken cotton swab or shaven toothpick).
CRITICAL: Pressing the coverslip prevents the spread of the superglue under the glass (Figure 3D). Spread of glue under the glass, despite applying pressure on it, usually indicates too thick and uneven bone around the craniotomy. This will obscure and/or reduce the imaging area. Additionally, it might lead to fast bone re-growth. Troubleshooting Problem 1.
CRITICAL: Make sure that the glass perimeter is completely sealed by adding extra drops of glue around the window coverslip without the pressure from above. Note, that the glue can be added on the dura directly (in case the craniotomy area is not fully covered by the coverslip; Figure 3D).
Fix the Headplate
Timing: 10 min
-
22.
Position the aluminum headplate over the coverslip parallel to the eye-line.
CRITICAL: It is important that the headplate is mounted at about the same level as the glass coverslip (Figure 2K). If the headplate rests much higher than the glass, the thinning of the skull (step 12; Figures 2E and 3B) was not enough. This might cause steric problems for imaging with bulky or short working distance objectives.
Note: The headplate and coverslip planes should be as parallel as possible.
Note: Make sure that the cut edge of the central hole is on the upper side (Figure 2K).
-
23.
Attach the headplate to the skull with a small drop of superglue.
-
24.
Prepare the dental acrylic according to the instruction of the producer.
-
25.
Glue the headplate with dental acrylic to the skull by filling all the space under and around the headplate, including the edges of the glass window and exposed skull (Figure 2L). To do this, as in step 21, pick up droplets of dental acrylic with a narrow but flat end wooden stick (for example, broken cotton swab or shaven toothpick).
CRITICAL: Cover all the exposed bone. Otherwise the open wound might get infected. Typically, the mouse will have to be removed from the stereotaxic frame toward the end, since the ear bars block the access to the space below the headplate. So, continue filling dental acrylic under the headplate with the mouse in your hand.
CRITICAL: The large area of bone covered with dental acrylic ensures strong fixation of the headplate. Otherwise, the headplate might tear off when the mouse is head-fixed. Troubleshooting Problem 4.
Other Steps during and after the Surgery
Timing: 1–5 min per step
These steps ensure animal welfare during and after the surgery as well as faster recovery.
-
26.
If the mouse shows reflexes or reactions to a procedure or signs of waking, add temporally 0.5%–1% isoflurane anesthesia. Typically, this additional anesthesia is applied during the drilling (step 12).
-
27.
During the surgery, inject saline (10–20 μL/g, s.c.) every hour to avoid dehydration.
-
28.
If medetomidine was used for anesthesia (step 2b), after the surgery, inject Atipamezole hydrochloride (0.3 μL/g, i.p.) for faster waking.
-
29.
Prior to waking, inject analgetic (here, Buprenorphine dissolved in 0.9% saline; 0.1 μg/g, s.c.) to ease the pain and so reduce distress.
-
30.
Add recovery food supplement (DietGel 76a, Clearh2o) to the cage.
-
31.
Check the mouse regularly in the days following the surgery for any sign of pain and omission of grooming. In such a case, inject analgetic (for example, Buprenorphine 0.1 μg/g, s.c.). Troubleshooting Problem 3.
-
32.
If the brain shows any sign of inflammation (foggy view, high autofluorescence) or blood under the window during the 2 weeks following the surgery, inject immunosuppressant (for example, Carprofen 10 μg/g body weight, i.p.) for 2–3 consecutive days to suppress the immune response. Troubleshooting Problem 1.
Expected Outcomes
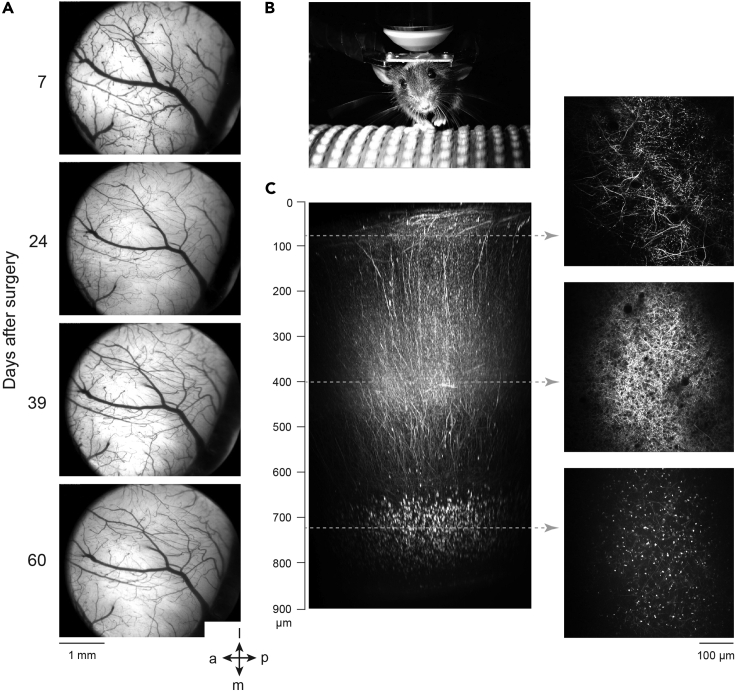
After some practice, the success rate of the surgery should be over 90% and the mouse should recover within 2–3 days. The window should always be clear and allow imaging of fluorescence (for example, retrograde tracer) or intrinsic signals within the first week after the surgery (Figure 4A). The start- as well as the end of imaging experiments using genetically encoded indicators is only restricted by the expression time-course of the fluorescent probes. The maximal time period for imaging should not be limited by bone re-growth due to the excessive thinning of the bone and the large craniotomy area. Here, we did not observe any signs of inflammation or bone re-growth at least for 2 months after the surgery (60 days, Figure 4A). The high-quality cranial window in combination with a well-adjusted two-photon microscope, allows deep in vivo imaging (Figure 4B) and imaging Ca2+ activity of the same layer 6 neurons in V1 for several hours continuously on several consecutive days (Augustinaite and Kuhn, 2020). As an example, we show a reconstruction of dLGN axons and beads retrogradely transported to L6 neurons, imaged with two-photon microscopy 55 days after the surgery (Figure 4C).
Figure 4.
Expected Outcomes of the Chronic Cranial Window Surgery
(A) Image of the cranial window 7–60 days after the surgery. The same mouse as in Figure 2.
(B) A mouse, head-fixed on the imaging setup.
(C) Two-photon images (right) and reconstruction of dLGN thalamocortical axons (labeled by AAV injection for TurboRFP expression in dLGN) as well as retrogradely transported beads (injected into dLGN) in cortical layer 6. The reconstruction spans full cortical depth (900 μm; left; adapted from Augustinaite and Kuhn, 2020). dLGN, dorsal lateral geniculate nucleus. The orientation: a, anterior; p, posterior; l, lateral; m, medial.
Limitations
This protocol is most suitable for cranial window installation over dorsal and dorsolateral brain areas. The brain area should have a curvature which can still be flattened by the glass coverslip. A craniotomy over lateral or ventral cortical areas as well as large and curved surfaces will require adaptation.
We recommend using 2–3 months old mice for the described surgery. Earlier, the skull grows fast, is flexible, and not calcified; therefore, it is difficult to fix the headplate and maintain a high-quality imaging window. After 4 months, mice develop a very thick skull; the surgery will take longer, which can lead to a longer recovery period or a higher death rate during or after the surgery.
Troubleshooting
Here, we summarize the potential problems which can occur during or after the procedure and suggested potential solutions.
Problem 1
Opaque cranial window (steps 4, 12, 15, 20, 21, and 32)
The transparency below the window defines the imaging quality. The transparency can be compromised by glue, blood, re-growth of bone below the glass and autofluorescence caused by a strong immune response. Especially, deep and/or peripheral imaging sites will be not accessible.
Potential Solution
To avoid such problems, be careful and patient throughout the surgery. Inflammation can be caused by over-heating and damage of the dura, blood vessels, and the brain itself when drilling. There is a risk of damage due to drilling not only at the edges of the craniotomy, but also over the more distant areas of the bone. In our experience, such damage is indicated by bruises seen through the bone over the thinned-skull area. Therefore, use a large-diameter drill bit, drill with smooth motion, keeping the drill at a shallow angle and applying minimal pressure with the drill bit. Avoid drilling for too long at the same position. Periodically cool and clean the bone with saline and compressed air.
Do not glue the window coverslip before the damaged blood capillaries are cured by cleaning the dura with saline or Carprofen-soaked gel-foam.
Do not leave a gap between the dura and the glass coverslip. This can be achieved by slight pressing in the center of the coverslip with a small cotton-tip if the craniotomy was made large enough and the surrounding bone was thinned. Add a drop of saline before placing the coverslip, if necessary. Otherwise, the glue might leak below the glass or the empty space will fill with cerebrospinal fluid and/or blood when the brain volume increases after anesthesia wears off.
If the window does not clear within 2–3 weeks after surgery, injections of Carprofen (dissolved in 0.9% saline; 10 μg/g body weight, i.p.) for 2–3 consecutive days might help to suppress the immune response and cease the signs of inflammation.
Re-growth of bone can be prevented by making a large craniotomy and by rigorous but careful thinning of the bone around it as well as thorough cleaning of the skull (while drilling) and dura (after the craniotomy) from the drilled bone particles. The thin bone at the edges of the craniotomy will be completely covered with glue when fixing the coverslip. This will prevent the re-growth from the periphery. Meanwhile, the cleaning of the bone particles will prevent formation of bone “islands” below the glass.
Problem 2
Dura and brain damage when thinning or removing the bone (steps 11, 12, and 14)
Large-scale bleeding and brain damage will occur if the drill bit breaks through the bone while drilling. Mechanical dura and brain damage can also be caused by sharp edges of the bone while performing the craniotomy. In such cases, the mouse must be sacrificed immediately.
Potential Solution
Be very careful when using small diameter drill bits or when dealing with the thin bone (for example, when thinning the bone, especially in young animals). Use a large (1–1.2 mm) diameter drill bit and hold it at a shallow angle while drilling to minimize the risk of the breaking through the bone.
Even though extensive bone thinning around the craniotomy is potentially dangerous, it is highly recommended. The thin bone does not break, but tears during the craniotomy, leaving dura and brain intact (Figures 3B and 3C). This is especially important if the craniotomy is performed near large blood vessels, such as the transverse sinus (Figure 3; see also Troubleshooting Problem 3).
Problem 3
Blood loss (steps 11, 12, 14, and 31)
The mouse can die during or after the surgery because of blood loss due to injury of large blood vessel while drilling or opening the skull. This is of especially high risk if the craniotomy must be made near or above a large blood vessel or sinus located under the sutures of the skull.
Potential Solution
Avoid large blood vessels at the edges of the craniotomy area, since this place requires most of the drilling to make the thinnest bone. If possible, maintain large vessels either inside or well outside when defining the craniotomy area. Maximally thin the bone around the craniotomy area, so that the bone would not break and, therefore, would not damage dura, brain, or large blood vessels during the craniotomy.
Check the recovery of the mouse regularly during the days following the surgery for any sign of pain and omission of grooming, especially in case of strong bleeding during the surgery. Inject analgetic drugs (for example, Buprenorphine 0.1 μg/g, s.c.), to ease the pain and as a source of water.
Problem 4
Headplate comes off (steps 10, 12, and 25)
The headplate, together with the dental acrylic-attached bone, might tear off when the mouse is head-fixed and awake. The risk of the headplate coming off is especially high if the craniotomy is at an anterior (as olfactory bulb or frontal cortical regions) or posterior (as V1 or cerebellum) location so that the mouse has a strong leverage on the headplate fixation. In such a case, the mouse must be sacrificed immediately.
Potential Solution
An insufficient amount of dental acrylic bonding the headplate to the bone is the most frequent cause of the headplate coming off. Make sure to expose a large area of the skull by making a long incision and by removing skin. Additionally, drill away some bone even on most anterior/posterior parts of the exposed skull, since the dental acrylic bonds better to the rough surface.
Problem 5
Failure of labeling (steps 16–18)
The most common failures when no labeling is observed results from (i) missing the injection target, or (ii) too dense labeling along the pipette tract so that the desired labeling gets obscured.
Potential Solution
Wrong zeroed bregma coordinates are the most likely cause of labeling failure, when the injection target is relatively small and therefore high precision is required. Careful leveling of the skull on sagittal and horizontal planes, as well as adjusting the ventral distance after the bone thinning are necessary for accurate injections. Also, the age and gender of the mouse should be considered if a systematic offset persists. As a rule of thumb, younger animals have thinner skulls and slightly smaller brains than older ones; and female mice have slightly thinner (our observation), smaller and different shape skulls (Maga et al., 2015) than males of the same age.
During or after the injection, fluorescent particles, dyes, or viral vectors might spread up along the pipette track instead of into the tissue at the injection point, following the path of least resistance. To prevent such unwanted spread, wipe the pipette tip with a PBS-soaked sponge before inserting it into the brain. Wait 3–5 min after the insertion allowing the tissue to settle and seal around the pipette. Inject under visual observation of the meniscus, to control the injection volume as well as speed and wait for 3–5 min before retracting the pipette. This waiting period is necessary to make sure that the injected liquid penetrates the tissue instead of filling the gap left by the retracting pipette.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Sigita Augustinaite (sigita.augustinaite@oist.jp).
Materials Availability
This study did not generate new reagents.
Data and Code Availability
This study did not generate any data, sophisticated custom computer code, or algorithm.
Acknowledgments
This work was supported by JSPS KAKENHI grant number 17K14941 to S.A. and the OIST Graduate University. This protocol was developed in the AAALAC internationally accredited Animal Facility of OIST Graduate University.
Author Contributions
Conceptualization, S.A. and B.K.; Methodology, S.A. and B.K.; Investigation and Visualization, S.A.; Writing – Original Draft, S.A.; Writing – Review & Editing, S.A. and B.K.; Funding Acquisition, S.A. and B.K.; Supervision, S.A.
Declaration of Interests
The authors declare no competing interests.
References
- Augustinaite S., Kuhn B. Complementary Ca(2+) activity of sensory activated and suppressed layer 6 corticothalamic neurons reflects behavioral state. Curr. Biol. 2020;30:3945–3960.e5. doi: 10.1016/j.cub.2020.07.069. [DOI] [PubMed] [Google Scholar]
- Dalphin N., Dorgans K., Khaskin E., Kuhn B. Voltage imaging of cortical oscillations in layer 1 with two-photon microscopy. eNeuro. 2020;7 doi: 10.1523/ENEURO.0274-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A., De Paola V., Wilbrecht L., Trachtenberg J.T., Svoboda K., Portera-Cailliau C. Imaging neocortical neurons through a chronic cranial window. Cold Spring Harb. Protoc. 2012;2012:694–701. doi: 10.1101/pdb.prot069617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maga A.M., Navarro N., Cunningham M.L., Cox T.C. Quantitative trait loci affecting the 3D skull shape and size in mouse and prioritization of candidate genes in-silico. Front. Physiol. 2015;6:92. doi: 10.3389/fphys.2015.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Franklin K.B.J. Academic Press; 2013. The mouse brain in stereotaxic coordinates. [Google Scholar]
- Roome C.J., Kuhn B. Chronic cranial window with access port for repeated cellular manipulations, drug application, and electrophysiology. Front. Cell Neurosci. 2014;8:379. doi: 10.3389/fncel.2014.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roome C.J., Kuhn B. Simultaneous dendritic voltage and calcium imaging and somatic recording from Purkinje neurons in awake mice. Nat. Commun. 2018;9:3388. doi: 10.1038/s41467-018-05900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate any data, sophisticated custom computer code, or algorithm.