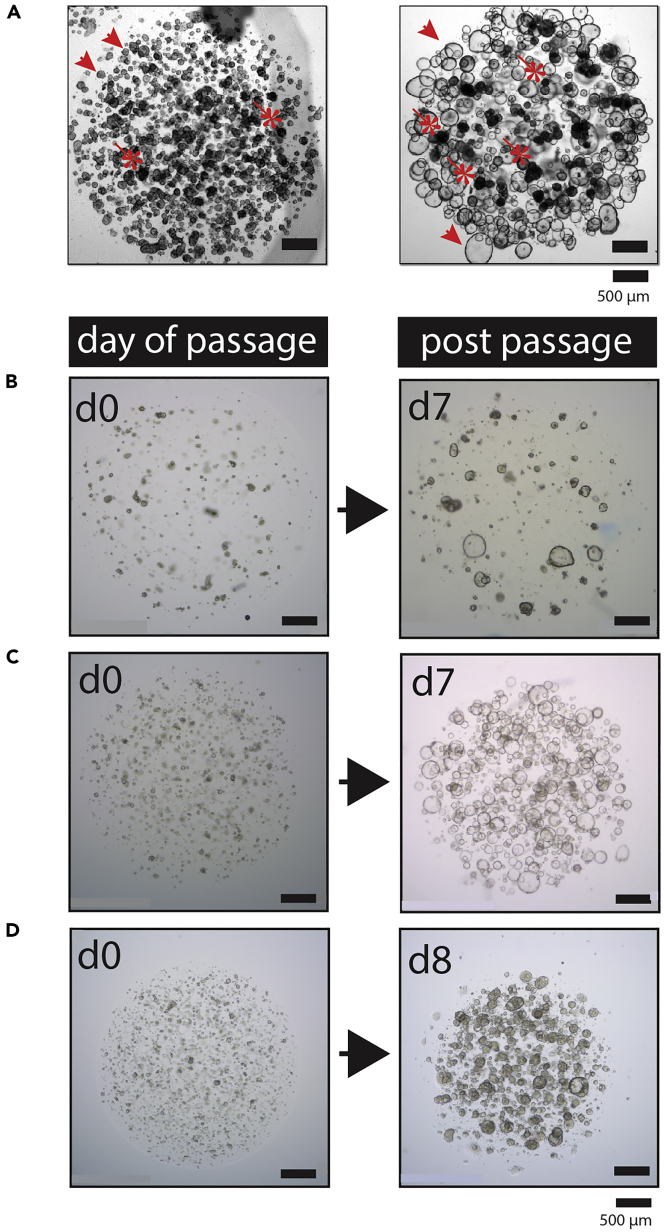
Figure 4.
Passaging of Organoid Cultures
(A) Representative bright-field images of two organoid cultures that were plated too densely after passaging, resulting in death of organoid structures. Death can be observed mostly in the center of the BME/Matrigel dome, most likely because of lack of nutrients (that diffuse into the droplet from the media) and oxygen. Live organoids (enriched on the side of the drops) are indicated by arrows, whereas dead organoids (enriched on the center of the drops) are indicated with asterisks. Scale bar, 500 μm.
(B–D) Representative bright-field microscopy images of three different organoid cultures split for passaging. The left panels show the appearance of the cultures right after shearing (d0). The right panels show the appearance of the cultures few days later (7–8 days) and right before they will be passaged.
(B) Representative bright-field image of a culture that was plated with a density that was too low, as can be seen by the limited number of organoids that grew out after 7 days in culture. Here, the organoid culture was pooled (the material was collected and replated in a smaller volume of BME) to assure that organoids were in close proximity to each other. After that, when organoids grew big, the culture could be passaged again.
(C and D) Representative images of a cystic (C) and dense (D) organoid culture that were split and subsequently passaged at a correct density. This density allowed efficient outgrowth of the organoids, without the cell death shown in Figure 4A as can be observed in the right-side panels. Scale bar, 500 μm.