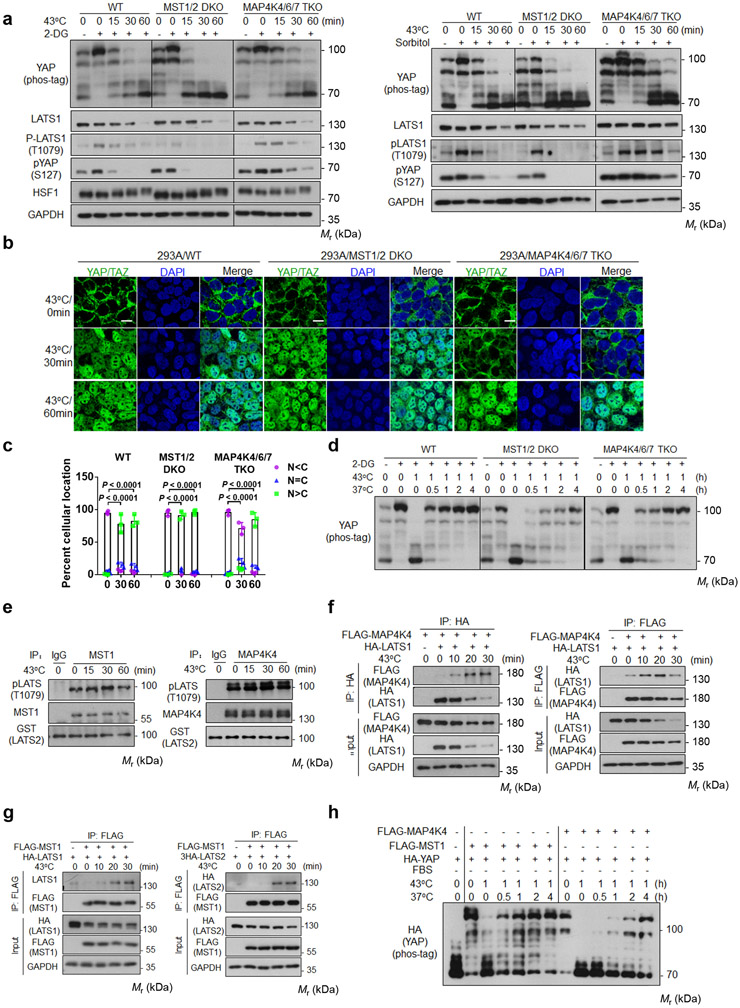
Figure 3. MST1/2 and MAP4Ks are not required for heat shock-induced YAP regulation.
(a) Deletion of MAP4Ks, but not MST, slightly delays heat shock-induced YAP dephosphorylation. HEK393A WT, MST1/2 DKO, and MAP4K4/6/7 TKO cells under medial confluence were pretreated with 2-DG (left panels) or sorbitol (right panels) and then subjected to heat shock. The phosphorylation of YAP and LATS1 were analyzed by Western blot.
(b) MAP4K4/6/7 knockout delays YAP nuclear localization. Cells were stained for YAP/TAZ for immunofluorescent microscopy. Representative pictures from three independent samples are shown. Scale bars,10 μm.
(c) Quantification of YAP/TAZ nuclear and cytosolic localization. Data are mean ± s.d.; n = 3 biologically independent samples. Two-way ANOVA test.
(d) MST1/2 knockout delays recovery after heat shock. HEK293A WT, MST1/2 DKO, and MAP4K4/6/7 TKO cells were pretreated with 2-DG, then subjected to heat shock for 1 h followed by recovery at 37°C for the indicated durations.
(e) Heat shock does not affect MST1 and MAP4K4 kinase activity. Endogenous MST1 (left panel) or MAP4K4 (right panel) immunoprecipitated from heat shocked HEK293A cells was assayed using GST-LATS2 as a substrate. LATS2 phosphorylation was determined with pLATS (Thr1079) antibody.
(f) Heat shock increases LATS and MAP4K4 interaction. HEK293A cells were co-transfected with FALG-MAP4K4 and HA-LATS1. 24 h after transfection, cells were subjected to heat shock. HA (left panel) and FLAG (right panel) antibodies were used for immunoprecipitation and the co-precipitated proteins were detected by Western blot.
(g) Heat shock increases LATS and MST1 interaction. Experiments were similar to panel f except cells were co-transfected with FLAG-MST1 and HA-LATS1 (left panel) or 3×HA-LATS2 (right panel).
(h) YAP re-phosphorylation time course in MST1-rescued or MAP4K4-rescued MM8KO cells after shifting back to 37°C. Plasmids for HA-YAP and FLAG-MST1 or FLAG-MAP4K4 were co-transfected into HEK293A MM8KO cells. One day after transfection, cells were subcultured to new plates and reached medial confluence the next day, serum starved for 2 h, then subjected to heat shock at 43°C for 1 h followed by recovery at 37°C for the indicated durations. Immunoblotting in panels a and d-h has been performed two times with similar results. Source data are available online.