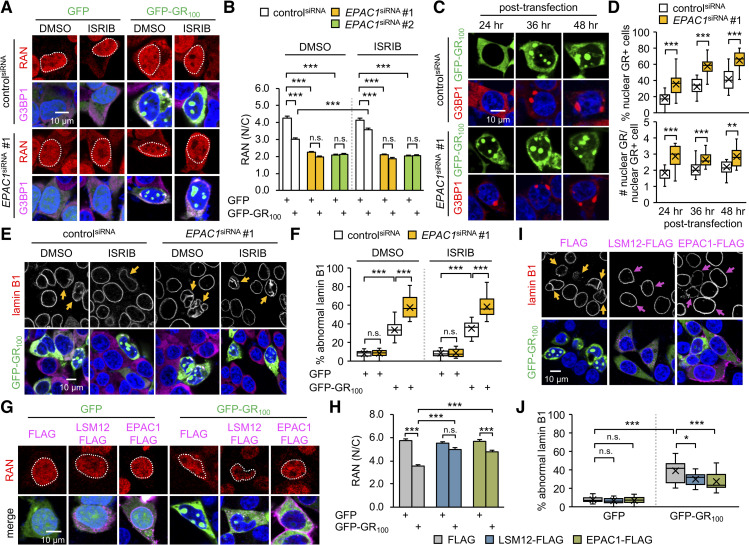
Fig 6. EPAC1 suppresses poly(GR) toxicity relevant to NCT and nuclear integrity.
(A) SH-SY5Y cells were transfected with controlsiRNA or EPAC1siRNA 24 hours before transfecting with an expression vector for GFP or GFP-GR100. Transfected cells were co-stained with anti-RAN antibody (red), anti-G3BP1 antibody (magenta), and Hoechst 33258 (blue) 72 hours after siRNA transfection. Where indicated, cells were treated with 2-μM ISRIB or DMSO (vehicle control) for 5 hours before antibody staining. (B) The nucleocytoplasmic RAN gradient was quantified as in Fig 2D. Two-way ANOVA detected significant interaction effects of GFP-GR100 and ISRIB treatment on the RAN gradient in controlsiRNA cells (P = 0.0008), but not in EPAC1siRNA cells (P = 0.4674 for EPAC1siRNA #1; P = 0.7870 for EPAC1siRNA #2); significant interaction effects of GFP-GR100 and EPAC1 depletion on the RAN gradient regardless of ISRIB treatment (P < 0.0001 for both EPAC1siRNA in DMSO; P = 0.0327 for EPAC1siRNA #1 in ISRIB; P = 0.0013 for EPAC1siRNA #2 in ISRIB). Data represent means ± SEM (n = 124–145 GFP–or GFP-GR100–positive cells from 3 independent experiments). n.s., not significant; ***P < 0.001, as determined by Tukey post hoc test. (C) EPAC1 depletion facilitates the nuclear accumulation of poly(GR) protein. SH-SY5Y cells were co-transfected with each siRNA and a GFP-GR100 expression vector as above. Transfected cells were co-stained with anti-G3BP1 antibody (red) and Hoechst 33258 (blue) at the indicated time points after plasmid DNA transfection. (D) The assembly of nuclear poly(GR) granules was quantified as in Fig 1. Data represent means ± SEM (n = 18–19 confocal images obtained from 3 independent experiments; n = 366–413 GFP-GR100–positive cells). **P < 0.01, ***P < 0.001, as determined by Student t test. (E) EPAC1 depletion exacerbates the poly(GR)-induced disruption of the nuclear lamina. SH-SY5Y cells were co-transfected with each siRNA and a GFP-GR100 expression vector, treated with 2-μM ISRIB or DMSO (vehicle control) and then co-stained with anti-lamin B1 antibody (red), anti-G3BP1 antibody (magenta), and Hoechst 33258 (blue) as described above. Yellow arrows indicate GFP-GR100–positive cells with severe nuclear lamina disruption. (F) The abnormal nuclear laminar morphology was quantified as in Fig 3E. Two-way ANOVA detected significant interaction effects of GFP-GR100 and EPAC1 depletion on the nuclear integrity regardless of ISRIB treatment (P < 0.0001 for both DMSO and ISRIB). Data represent means ± SEM (n = 15 confocal images obtained from 3 independent experiments; n = 313–545 GFP–or GFP-GR100–positive cells). n.s., not significant; ***P < 0.001, as determined by Tukey post hoc test. (G, H) Overexpression of LSM12 or EPAC1 suppresses the poly(GR)-induced disruption of the RAN gradient. SH-SY5Y cells were co-transfected with different combinations of expression vectors for FLAG-tagged LSM12, FLAG-tagged EPAC1, and GFP-GR100. Transfected cells were co-stained with anti-RAN antibody (red), anti-FLAG antibody (magenta), and Hoechst 33258 (blue). The nucleocytoplasmic RAN gradient was quantified as in Fig 2D. Two-way ANOVA detected significant interaction effects of GFP-GR100 with LSM12 or EPAC1 overexpression on the RAN gradient (P < 0.0001 for both). Data represent means ± SEM (n = 101–109 GFP–or GFP-GR100–positive cells from 3 independent experiments). n.s., not significant; ***P < 0.001, as determined by Tukey post hoc test. (I, J) Overexpression of LSM12 or EPAC1 suppresses the poly(GR)-induced disruption of the nuclear lamina. Yellow arrows indicate GFP-GR100–positive cells with severe nuclear lamina disruption. Magenta arrows indicate cells overexpressing LSM12-FLAG or EPAC1-FLAG protein. The abnormal morphology of the nuclear lamina was quantified as in Fig 3E. Data represent means ± SEM (n = 11–19 confocal images obtained from 3 independent experiments; n = 235–508 GFP–or GFP-GR100–positive cells). n.s., not significant; *P < 0.05, ***P < 0.001, as determined by 2-way ANOVA with Tukey post hoc test. All underlying numerical values are available in S1 Data. ANOVA, analysis of variance; EPAC1, exchange protein directly activated by cyclic AMP 1; GFP, green fluorescent protein; ISRIB, integrated stress response inhibitor; LSM12, like-Sm protein 12; N/C, nuclear to cytoplasmic; NCT, nucleocytoplasmic transport; SEM, standard error of the mean; siRNA, small interfering RNA.