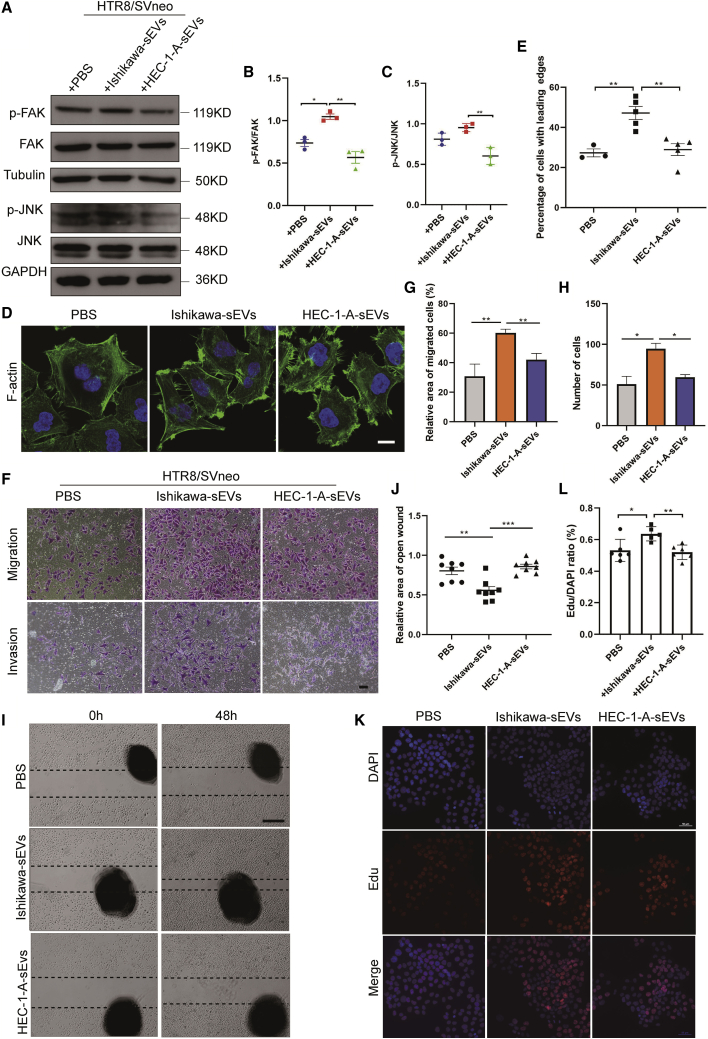
Figure 3.
Endometrial epithelial cell-derived sEVs affect embryo implantation
(A–C) HTR8/SVneo trophoblasts were serum starved and treated with PBS, Ishikawa-sEVs, or HEC-1-A-sEVs. After 48 h, the cells were immunoblotted for phosphorylated FAK (P-FAK) and P-JNK. The blots were also detected for total FAK, JNK, and tubulin or GAPDH. The ratio of phospho protein was measured and calculated using phospho protein/total protein. (D) HTR8/SVneo trophoblasts were incubated with serum-free medium supplemented with PBS, Ishikawa-sEVs, or HEC-1-A-sEVs for 12 h, and then the HTR8/SVneo cells were stained for F-actin using FITC-conjugated phalloidin. The white arrows show leading edges. Bar, 20 μm. (E) The percentages of cells with leading edges were determined. (F) Migration and invasion assay of HTR8/SVneo cells treated with PBS, Ishikawa-sEVs, or HEC-1-A-sEVs using Transwell. Bar, 50 μm. (G and H) Migrated and invaded cells were counted, and representative images were shown. (I) Images of wound-closure assays performed on HTR8/SVneo cells cultured in serum-free medium supplemented with PBS, Ishikawa-sEVs, or HEC-1-A-sEVs for 48 h. The dashed lines indicate the width of the wound. Bar, 500 μm. (J) The relative areas of an open wound in (I) were quantified and plotted. (K) The proliferation assay of HTR8/SVneo cells measured by EdU assay. (L) Quantitative analysis of proliferation in (K) (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001).