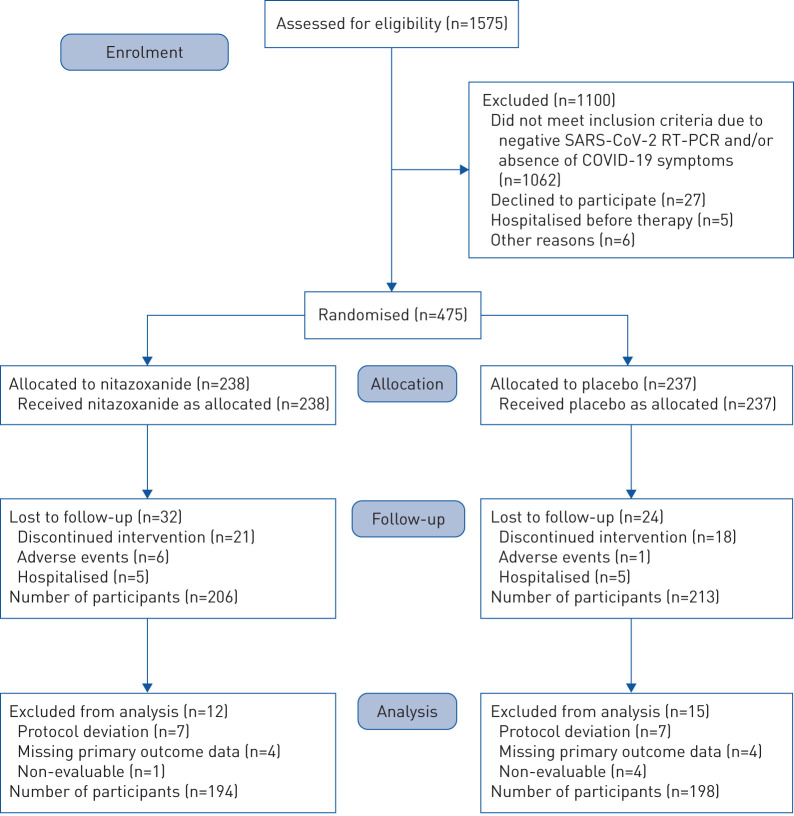
FIGURE 2.
Enrolment, randomisation, follow-up and treatment. 1575 patients were assessed for eligibility at the study sites. Of these, 475 tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection by reverse transcriptase (RT)-PCR and underwent randomisation. Reasons for exclusion before randomisation included negative RT-PCR collected at day 0 (baseline) and/or absence of coronavirus disease 2019 (COVID-19) symptoms (n=1062), refusal to participate (n=27), hospitalisation before the first dose of therapy (n=5) and other reasons (n=6). After randomisation (n=475), patients were excluded due to discontinued intervention (n=39), moderate adverse events (n=7) (all gastrointestinal upset) and hospitalisation (n=10) (n=5 patients from each group, none of whom completed therapy). During analysis, 12 patients were excluded from the nitazoxanide arm and 15 from the placebo arm due to protocol deviation, missing data on the primary outcome or non-evaluability, resulting in a studied population of 392 patients (194 in the nitazoxanide arm and 198 in the placebo arm).