Abstract
This chapter presents a description of standardized techniques used routinely in our laboratory to encapsulate different cell types using the alginate-PLL-alginate immunoisolation system. Given the importance of noninvasive tracking of encapsulated cell transplants, we present a detailed guidance to achieve maximum efficiency and functionality of the capsule preparations for optimal tracking posttransplantation. The provided protocols cover tracking of encapsulated cells using magnetic resonance (MR), X-ray, computed tomography (CT), and ultrasound (US) imaging. Practical suggestions to optimize each method with specific references to recommended suppliers are included.
Keywords: Alginate, Encapsulation, Noninvasive tracking, MRI, X-ray, Ultrasound
1. Introduction
Encapsulation of cells for transplantation purposes is a technique intended to protect cells from the innate and adaptive immune system of the host, while allowing diffusion of essential nutrients and oxygen required for graft survival [1, 2]. Encapsulation of cells is an established technique, first described by Lim and Sun in 1980 [3]. Since then, it has been applied with different rates of success in different laboratories [4–7]. In the case of allografts and xenografts, encapsulation can minimize or eliminate the need for immunosuppression thereby sparing the transplant recipient from the deleterious effects of immunosuppressive agents [8]. The use of xenografts is especially attractive due to the shortage of allograft tissue available [9]. Encapsulation has been proposed for the treatment of different diseases, such as dwarfism, hemophilia, kidney and liver failure [10–12], stroke, myocardial infarction [13], and diabetes [9, 14–16]. A commonly used method for immunoisolation is the encapsulation of islets in alginate membranes [17–19]. Alginate is a biocompatible polymer derived from purified algae that allows selective diffusion of molecules under a certain molecular weight. Many groups have applied polycations, such as poly-l-lysine (PLL), to control the pore size and provide stability to the capsules [20–22].
Despite it widely being used, many issues remain unresolved with microencapsulation. Among those issues, the best mode of delivery, initial engraftment and tissue biodistribution, and stability of the capsules over time require special attention [23]. Therefore, a method with which one can noninvasively image the fate of the capsules is needed [23–27]. This chapter focuses on several methods that are used in our lab to track microencapsulated cells using MRI, X-ray, CT, and US imaging [24].
2. Materials
2.1. Reagents
Primary cells, cell clusters, cell lines, or spheroids from the tissue of interest.
Cell culture medium (any appropriate culture medium for the cells being used).
10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer.
PRONOVA UP LVG ultrapure low-viscosity high-guluronate alginate (NovaMatrix, cat. no. 4200001) (see Note 1).
PRONOVA UP LVM ultrapure low-viscosity high-mannuronate alginate (NovaMatrix, cat. no. 4200201) (see Note 1).
Poly-l-lysine (PLL).
Barium sulfate.
Bismuth sulfate.
Superparamagnetic iron oxide (SPIO) particles (see Note 2)
PFOB (C8F17Br) (Apollo Scientific, cat. no. PC6167).
Perfluoro-15-crown-5-ether (C15F30O5).
Soy lecithin.
Safflower oil USP.
Calcium chloride, anhydrous powder.
Sodium citrate.
0.9 % sodium chloride solution.
Fluorescein diacetate (FDA, Sigma, cat. no. F7378).
Propidium iodide (PI, Invitrogen, cat. no. P3566).
2.2. Equipment
Nano syringe pump (Cole-Parmer, cat. no. EW-74902–00).
Ring stand.
Three-prong extension clamps.
High-voltage power supply (Bertran, model no. 230–30R).
Sonicator.
Electrical tape.
Automotive hookup wire.
Becton-Dickinson tuberculin syringe—1 ml only with slip tip.
Blunt needles (24G × 1/2-in., 20G × 1/2-in.).
Three-way stopcock with swivel male Luer lock (Medex, cat. no. MX5311L).
Millipore filter flask (0.2 μm).
Nylon filter (0.2 μm).
Corning 100 × 20 mm untreated culture dish.
Serological pipettes.
Erlenmeyer screw cap flask.
Ceramic stirring hot plate.
9.4 T horizontal bore magnet (Bruker BioSpin, Karlsruhe, Germany).
40-MHz Vevo 660 scanner (Visual Sonics, Toronto, Ontario, Canada).
2.3. Buffer and Solutions
Primary LVG alginate (2 % w/v): 10 mM HEPES buffer, Pronova UP LVG alginate.
Secondary LVM alginate (0.15 % w/v): 10 mM HEPES buffer, Pronova UP LVM alginate.
Poly-l-lysine solution (0.05 % w/v): 10 mM HEPES buffer, PLL.
Gelation bath: 100 mM calcium chloride, 10 mM HEPES buffer.
Superparamagnetic iron oxide (SPIO): 10 mg Fe/ml, saline or HEPES buffer (see Note 2).
Perfluorocarbon emulsion. 5 % lecithin (w/v), 2 % safflower oil (v/v), water.
3. Methods
3.1. Solutions
Primary LVG alginate (2 % w/v): Prepare a sterile solution of 10 mM HEPES-buffered normal saline (sterilize by filtration through a 0.2 μm filter), and pour the sterilized solution into a flask under sterile conditions. Add a stir bar to the flask and place it on a stirring hot plate with the heater turned off. Slowly add the LVG alginate (see Note 3). Place a screw cap on the flask and allow it to rotate overnight in a tissue culture hood with the UV light turned off. Alginate aliquots (see Note 4) can be stored at 2–4 °C for up to 1 month. Remove necessary aliquots from refrigeration the day before encapsulation and allow them to return to room temperature (20–25 °C).
Secondary LVM alginate (0.15 % w/v): Prepare secondary alginate in a manner identical to that for the primary alginate using Pronova UP LVM; store aliquots at 2–4 °C for up to 1 month.
Poly-l-lysine solution (PLL, 0.05 % w/v): Dissolve PLL in 10 mM HEPES-buffered normal saline and filter-sterilize the solution with a filter flask. Store aliquots at 2–4 °C for up to 1 year.
Gelation bath: Prepare a solution of 100 mM calcium chloride in 10 mM HEPES-buffered normal saline and filter-sterilize the solution with a filter flask. Store aliquots at 2–4 °C for up to 1 year.
3.2. Electrostatic Droplet Generator Set-Up
Cut two pieces of 5 ft. connection wire and strip 1 in. of insulation tubing off the distal ends of both wires. Attach one end of the wire to a suitable ground (e.g., a ring stand placed on the floor) and place the other end of the wire into a Petri dish containing the cationic gelation bath. Attach the other wire to the ground on the high-voltage generator and connect the other end of the wire to a separate ground (e.g., the inner metal wall of a tissue culture hood).
Pass a 20G 1/2-in. blunt needle through the insulation wall of the wire through wire braids and out the opposite insulation wall of the output wire of the high-voltage droplet generator.
Gas-sterilize the set-up using ethylene oxide gas or, alternatively, use a UV light assembly for sterilization.
Place a culture dish below the needle and ensure that it is properly grounded (e.g., by running a wire from the encapsulation bath out of the tissue culture hood and onto a ring stand on the ground, ensuring that the proximal portion of the wire in the gelation bath and hood remains sterile).
Just before encapsulation, add 50 ml of gelation solution to the culture dish (see Note 5, Fig. 1).
Fig. 1.
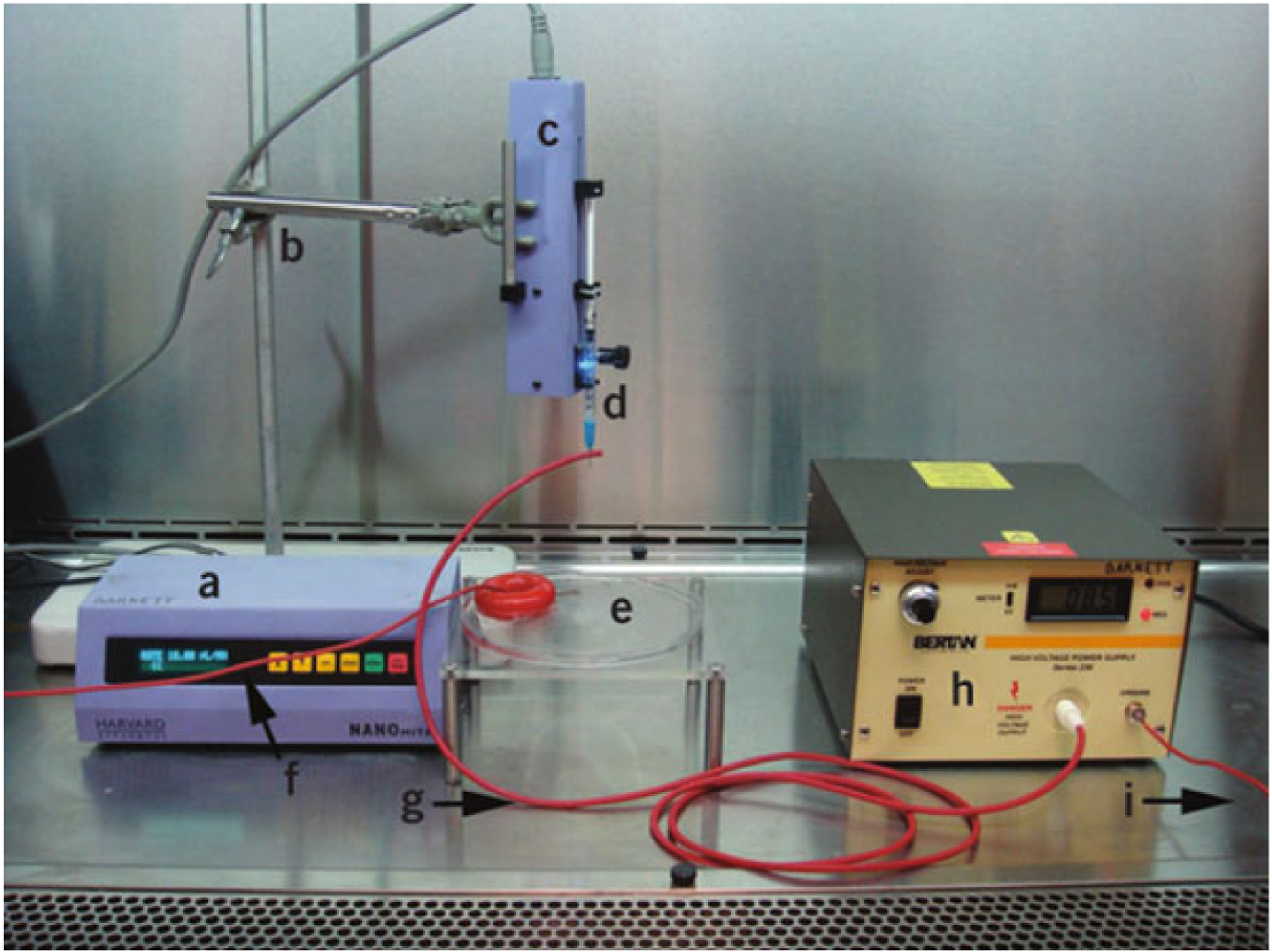
Essential capsule synthesis equipment. Syringe pump control box (a), ring stand and clamp (b), syringe pump remote injector (c), syringe with blunt-tip needle through the high-voltage output wire (d), gelation bath (e), gelation bath ground wire (f), high-voltage output wire (g), high-voltage generator (h), and high-voltage generator ground wire (i). Reprinted with permission from ref. 24. Copyright © 2011, Nature Publishing Group
3.3. Cell Encapsulation
Affix a blunt-tip needle with the wire from the high-voltage generator to the syringe containing the suspension of alginate, contrast, and cells.
Place the syringe with the needle attached in the syringe pump and adjust the height between the needle and the cationic (calcium or barium) solution by adjusting the ring stand. The distance should be approximately on the order of an inch (this results in a voltage differential of ~8 kV).
Turn on the high-voltage generator. Adjust the current before-hand, using an alginate solution without cells, to obtain the finest stream (and thus the smallest droplets) possible.
Preset the microinjector to inject a volume of 1 ml at a rate of 200 μl/min.
Ensure that the gelation bath is properly grounded and start the syringe pump (see Notes 6 and 7).
Allow microcapsules to remain in the gelation solution for 5 min.
Use a 10 ml serological pipette to remove microcapsules from the gelation bath; add them to a 50 ml conical tube.
Remove calcium chloride solution by allowing microcapsules to settle to the base of the conical tube, and then, carefully aspirate the entire volume of calcium chloride solution (Fig. 2a).
Fig. 2.
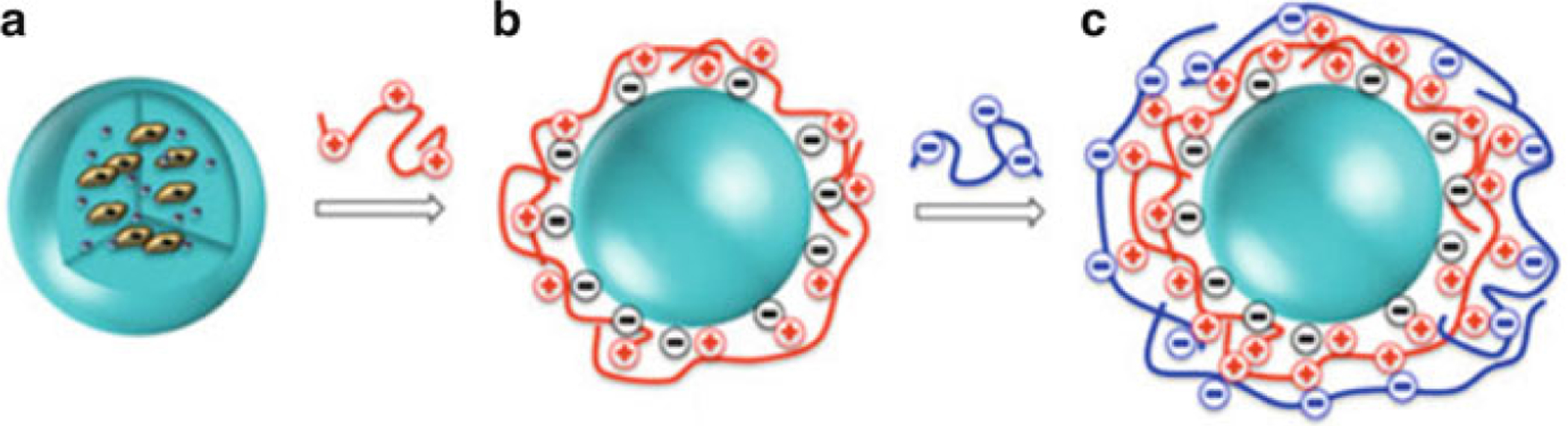
Synthesis of alginate-PLL-alginate capsules. (a) Schematic drawing showing microencapsulation of cells and contrast agents. The first alginate (LVG) layer of the microcapsule is cross-linked with PLL as a poly-cationic polymer (b, red) in step 3, and is further cross-linked with low-viscosity mannuronate (LVM) alginate (c, blue) in step 4. Adapted with permission from ref. 23. Copyright © 2012 John Wiley & Sons, Ltd
3.4. Application of Polycation Coat
Rinse microcapsules three times with 25 ml of 0.9 % (w/v) saline solution. Remove the solution for each change, as described in Subheading 3.3, step 8.
Add 40–50 ml of the PLL 0.05 % solution to microcapsules in the 50 ml conical tube.
Seal the conical tube and tape it horizontally onto a platform rocker. Rock at medium speed for exactly 6 min (Fig. 2b).
3.5. Application of Secondary Alginate Layer
Rinse microcapsules three times with 25 ml 0.9 % (w/v) saline solution.
Thoroughly remove the saline solution and add 40–50 ml of secondary alginate, prepared as described above, to the microcapsules in a 50 ml conical tube.
Seal the conical tube and tape horizontally onto a platform rocker. Rock at medium speed for 3 min.
Rinse the microcapsules again, three times with 25 ml 0.9 % (w/v) saline solution. Immediately transplant or place encapsulated cells in appropriate culture conditions [28] (Fig. 2c).
3.6. Incorporation of Contrast in Primary Alginate
Follow the next steps for MR contrast, X-ray contrast, and trimodal (perfluorocarbon) contrast.
- MR contrast agent: magnetocapsules (see Note 8) [29–31].
- To create MR-visible alginate capsules, mix 20 % (v/v) SPIO with 80 % (v/v) primary alginate.
- For best results, load alginate and SPIO into two separate syringes and connect the syringes to a three-way stopcock; carefully remove any excess air.
- Once air is removed, move the stopcock to the closed position and mix the contents of the two syringes back and forth until a homogenous solution is created.
- X-ray imaging agent (see Note 9) [32].
- To create X-ray-visible alginate capsules, first dissolve barium or bismuth sulfate into 0.9 % saline at a concentration of 50 mg/ml.
- Vortex this solution to break down any large aggregates.
- Mix this barium sulfate solution with primary alginate, as described in Subheading 3.6, steps 2b and 2c.
- Multimodal contrast agent: fluorocapsules (see Note 10) [33].
- To prepare fluorinated US-visible capsules, first prepare a perfluorocarbon emulsion and filter-sterilize it through a 0.2 μm nylon filter.
- Sonicate the lecithin/safflower oil solution at 40 % power on ice until the solution is almost transparent.
- Add perfluorocabon to sonicated lecithin solution at a concentration ranging from 12 % perfluorocarbon (v/v) to 40 % perfluorcarbon (v/v).
- Sonicate perfluorocarbon lecithin solution for 25 min on ice until a milky homogenous solution is formed. Leave fluorocarbons on ice until incorporation in the primary alginate layer.
- Emulsion should be incorporated into the alginate layer within 1 h of emulsion preparation, as described in Subheading 3.6, steps 2b and 2c.
3.7. Examples of X-ray Contrast, 1H MR Contrast, and 19F MR Contrast
Fluoroscopic imaging was performed using a Toshiba Infinix VC-i unit, with imaging settings of 64 kV peak, 66 ms exposure time, 112 mA tube current, and 910 mm SID [25] (Fig. 3).
1H MR-trackable capsules (Fig. 4): MR imaging was performed using a 9.4 T horizontal bore magnet with a custombuilt animal holder and a whole-body volume transmitting-receiving coil. The microcapsules were imaged by using T1-weighted spin-echo (rapid acquisition with relaxation enhancement) and T2*-weighted gradient-echo (gradient-echo flow compensated) sequences. The T1-weighted imaging parameters for the saline phantoms were 500/14.1 (repetition time msec/echo time msec), a field of view of 1.6 × 1.6 cm, a matrix of 256 × 256, a section thickness of 1 mm, and 24 acquired signals. The same parameters were used for T2*-weighted imaging, with the exception of an echo time of 6.7 ms and 12 acquired signals. The T1-weighted imaging parameters for the mice were 500/14.1, a field of view of 2.4 × 1.8 cm, a matrix of 256 × 192, a section thickness of 0.8 mm, and 20 acquired signals with fat suppression and zero filling = 512 × 512 (matrix size). Micro-CT of the phantoms and mice was performed by using an X-SPECT Gamma Medica imager with tube settings of 75.16 kV and 240.3 mA, a 64-mm detector setting, and 512 projections [34].
19F-MR imaging (Fig. 5): MR imaging was performed placing 1–50 PFPE capsules in 0.2 ml polymerase chain reaction tubes and covered with 4 % gelatin. This phantom was imaged by using a clinical 3.0 T imager. To this end, a custom fluorine phased-array coil with four coil elements was used. Scout proton MR images were obtained by using a surface coil (Body Matrix; Siemens) followed by manual shimming by using the body coil. The custom fluorine coil was then used to obtain fluorine images by using a three-dimensional true fast imaging with steady-state free precession pulse sequence with the following parameters: 4.1/2.1; number of signals acquired, 32; 1.5 × 1.5 × 2.0 mm voxel size; field of view, 672 × 250; 1002 Hz bandwidth; 70° flip angle; and an acquisition time of about 12 min [35].
Fig. 3.
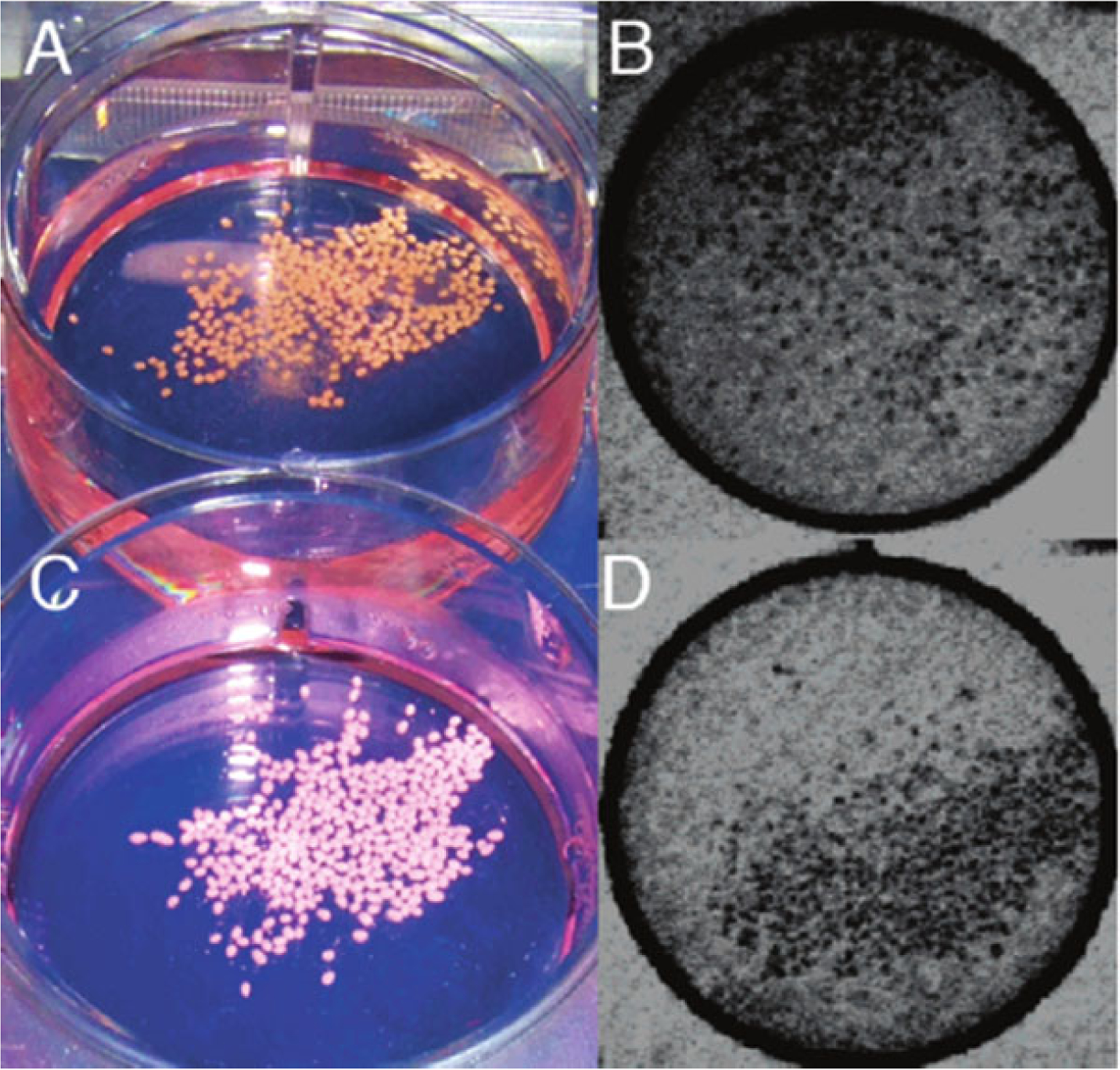
X-ray-trackable capsules. Macroscopic (a, c) and fluoroscopic (b, d) images of bismuth X-caps (a, b) and barium X-caps (c, d). Single capsules can be clearly identified. Reprinted with permission from ref. 25. Copyright© 2006, American Chemical Society
Fig. 4.
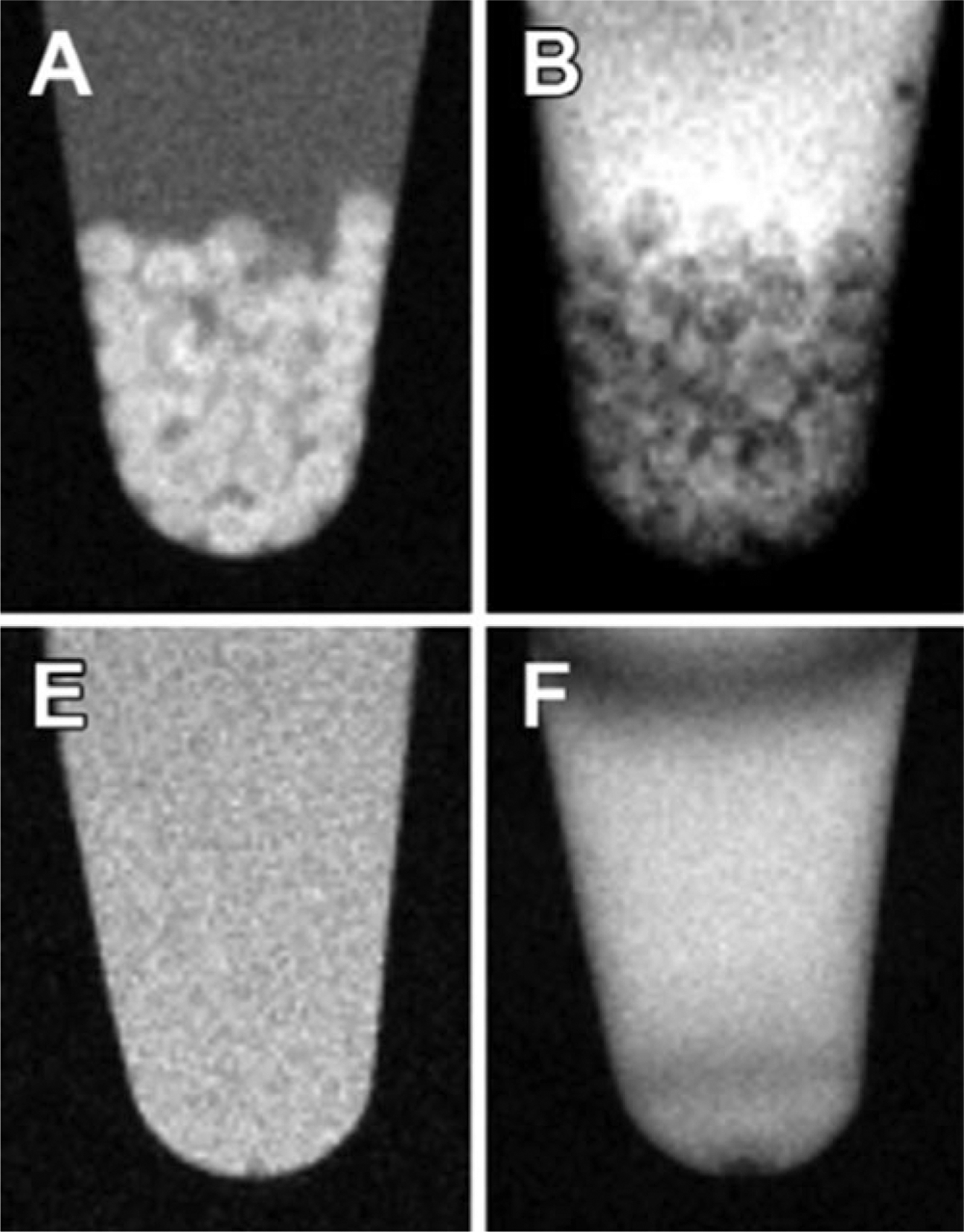
1H MR-trackable capsules. (a, e), T1-weighted and (b, f) T2*-weighted MR images of alginate-protamine sulfate-alginate (APSA) labeled with gadolinium-gold (GG) (top) and APSA without GG (bottom) microcapsules in saline. The appearances of the capsules in the MR imaging appear as hyperintense on the T1-weighted and as hypointense on the T2*-weighted images. Reprinted with permission from ref. 34. Copyright © 2015 Radiological Society of North America
Fig. 5.
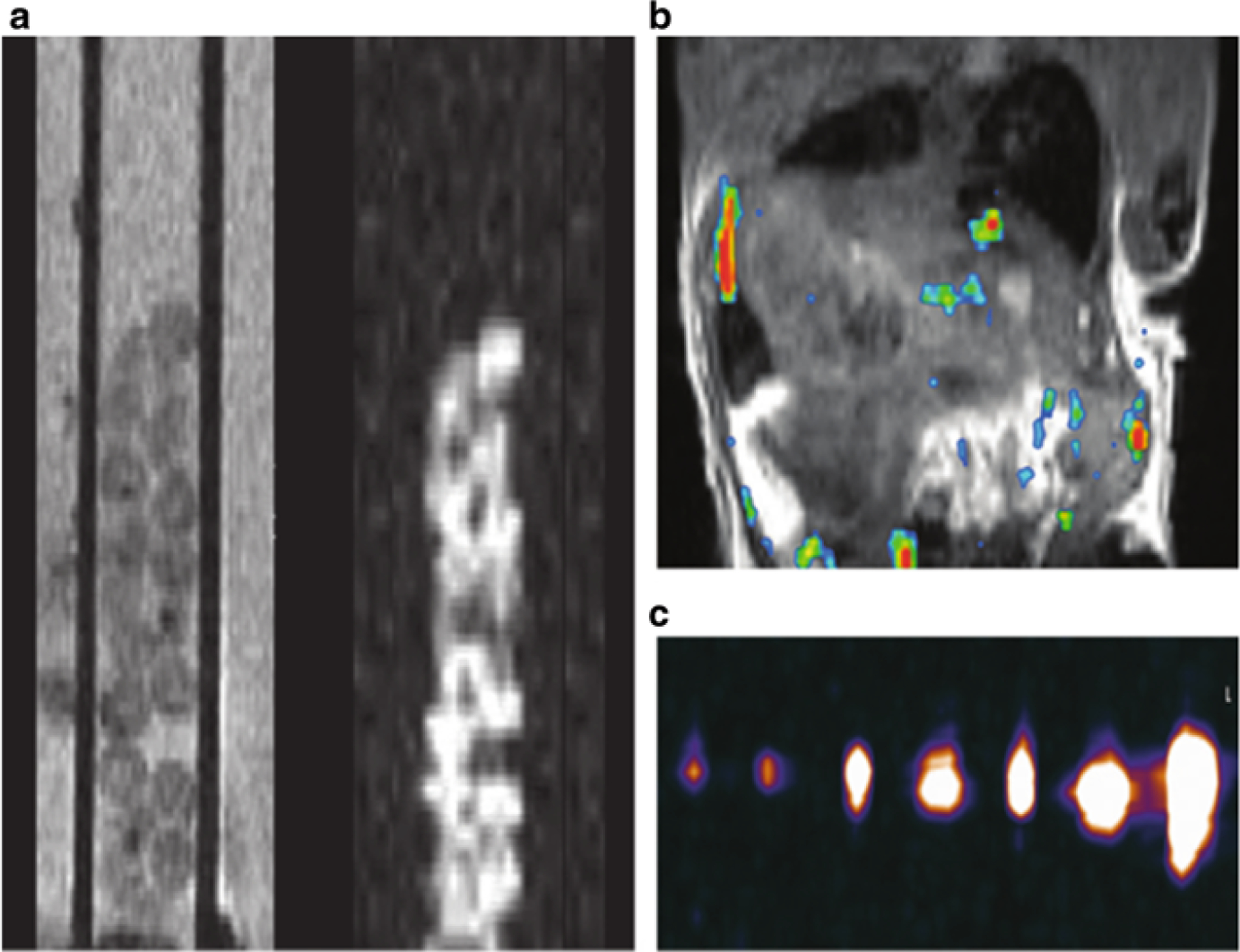
19F MR-trackable capsules. (a) In vitro proton T2-weighted MR image (left) and corresponding 19 F MR image (right) of fluorocapsules in a standard 5 mm glass tube, obtained with a 11.7 T preclinical system. (The capsules were introduced in a 500 mm capillary tube.) (b) In vivo 19 F MR image of a mouse after intraperitoneal transplantation of 2000 fluorocapsules. An overlay of the 19 F image (pseudocolor) on the 1H image (gray scale) is shown. (c) Fluorine 19 MR image of fluorocapsules obtained with a 3.0 T clinical system. From left to right, images are of gel phantoms containing 1, 2, 5, 10, 15, 25, and 50 capsules. Reprinted with permission from ref. 35. Copyright © 2015 Radiological Society of North America
3.8. Adding Cells to Primary Alginate/Contrast Solution
Pellet cells or allow clusters or spheroids of cells to settle in a 50 ml conical tube and remove all but 100 μl of culture medium. Depending on the expected growth of the cell population, the total concentration of cells should be adjusted to a concentration of 1–5 % (v/v) of that of the primary alginate layer. For growth-arrested cells, a higher number of cells can be encapsulated. For highly proliferative cells, such as immortalized cell lines, cells should be added at lower concentrations (see Note 11).
With the appropriate mixture of contrast and alginate, add the cells at the appropriate concentration to the alginate using a 50 ml conical tube. Use a 1 cc tuberculin syringe to homogenize the alginate/contrast solution with cells (see Note 12).
4. Notes
To avoid endotoxin contamination and potential activation of the cells in the microcapsule, ensure that the source of alginate has low endotoxin levels.
The market of commercially available SPIO formulations has seen several products being discontinued (e.g. Feridex®, Endorem®, or Resovist®). In principle, any SPIO formulation with a total hydro-dynamic diameter of less than 150 nm should be suitable.
To minimize the aggregation of alginate, slowly pour alginate while stirring.
As it is difficult to dissolve alginate in small volumes of liquid, it is better to prepare a larger volume and aliquot it into 15 ml conical tubes for later use.
Irregularity of the surface of the microcapsule is an indication that swelling has occurred in the saline bath before the cross-linking with PLL. Thus, cations (i.e., 1 mM CaCl2) should be added to the saline solution to prevent swelling during washes.
At this stage, some of the microcapsules might present a tear-drop shape due to a low concentration of alginate, and therefore, the amount of alginate should be increased. If the alginate stream sputters, then the concentration of the primary alginate must be decreased. If the needle becomes frequently clogged during the drip, this is an indication that the cell clusters are too large for the needle and a larger gauge should be used.
If microcapsules are too large, as determined using a phase-contrast inverted microscope, increase the distance between the needle tip and the gelling bath or increase the voltage from the generator or lower the concentration of the primary alginate. If microcapsules begin to adhere to each other as they are collecting directly beneath the tip of the needle in the gelling bath, a sterile stir bar can be placed in the gelation bath and placed on a stir plate.
If the bloom from the signal created by magnetocapsules is too large, then the concentration of SPIO in the capsules is too high and it should be reduced for field strengths greater than 1.5 T. The magnetic susceptibility induced by these capsules can be quantified using phase map cross-correlation detection and quantification, which automatically identifies the localization of engrafted magnetocapsules, estimating their volume, magnetic susceptibility, and magnetic moment.
As barium sulfate is in powder form, it can be added at the necessary concentration for different imaging modalities. For example, for CT imaging, a concentration of 5 % (w/v) is acceptable, whereas for detection on fluoroscopy, a concentration of >10 % (w/v) is required. Single Ba X-caps and Bi X-caps can be clearly identified in vivo in mice and rabbits.
Contrast agent leaching from the microcapsules is an indication that the agent is too small or not soluble in water, and therefore, it should be incorporated within a liposome carrier before mixing with alginate.
If the cell density is too low or too high, then different ranges of seeding densities should be attempted to find the best range for diferent cell lines. If low cellular viability is observed, this might be attributable to a lack of critical matrix components that be incorporated into the alginate. To improve the survival of cells, these components should not be in alginate solution too long prior to the formation of beads.
Appropriate cell concentrations vary depending on cell types. For human islets, one islet per capsule should be used. For single cells, e.g., mesenchymal stem cells, a maximum of up to 300 single cells can be encapsulated. The duration of this step must be minimized, to avoid large periods of hypoxia to the cells. If the viability of cells is reduced substantially at this step, one could supplement the primary alginate with glucose, amino acids, or other nutrients.
References
- 1.de Vos P, Spasojevic M, Faas MM (2010) Treatment of diabetes with encapsulated islets. Adv Exp Med Biol 670:38–53 [DOI] [PubMed] [Google Scholar]
- 2.Nafea EH, Marson A, Poole-Warren LA, Martens PJ (2011) Immunoisolating semi-permeable membranes for cell encapsulation: focus on hydrogels. J Control Release 154:110–122 [DOI] [PubMed] [Google Scholar]
- 3.Lim F, Sun A (1980) Microencapsulated islets as bioartificial endocrine pancreas. Science 210:908–910 [DOI] [PubMed] [Google Scholar]
- 4.de Haan BJ, Faas MM, Hamel AF, de Vos P (2006) Experimental approaches for transplantation of islets in the absence of immunosuppression In: Ford AM (ed) Trends in diabetes mellitus research. Nova Science Publisher, Inc., New York, pp 131–162 [Google Scholar]
- 5.de Vos P, Spasojevic M, de Haan BJ, Faas MM (2012) The association between in vivo physicochemical changes and inflammatory responses against alginate based microcapsules. Biomaterials 33:5552–5559 [DOI] [PubMed] [Google Scholar]
- 6.Paredes-Juarez G, de Haan B, Faas M, de Vos P (2014) A technology platform to test the efficacy of purification of alginate. Materials 7:2087–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uludag H, De Vos P, Tresco P (2000) Technology of mammalian cell encapsulation. Adv Drug Deliv Rev 42:29–64 [DOI] [PubMed] [Google Scholar]
- 8.Orive G, Santos E, Poncelet D, Hernández RM, Pedraz JL, Wahlberg LU et al. (2015) Cell encapsulation: technical and clinical advances. Trends Pharmacol Sci 36:537–546 [DOI] [PubMed] [Google Scholar]
- 9.Krishnamurthy NV, Gimi B (2011) Encapsulated cell grafts to treat cellular deficiencies and dysfunction. Crit Rev Biomed Eng 39:473–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ponce S, Orive G, Hernández R, Gascón AR, Pedraz JL, de Haan BJ et al. (2006) Chemistry and the biological response against immunoisolating alginate-polycation capsules of different composition. Biomaterials 27:4831–4839 [DOI] [PubMed] [Google Scholar]
- 11.Spasojevic M, Bhujbal S, Paredes G, de Haan BJ, Schouten AJ, de Vos P (2014) Considerations in binding diblock copolymers on hydrophilic alginate beads for providing an immunoprotective membrane. J Biomed Mater Res A 102:1887–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spasojevic M, Paredes-Juarez GA, Vorenkamp J, de Haan BJ, Schouten AJ, de Vos P (2014) Reduction of the inflammatory responses against alginate-poly-L-lysine microcapsules by anti-biofouling surfaces of PEG-b-PLL diblock copolymers. PLoS One 9:e109837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang X, Zhang X, Wang X, Wang C, Tang B (2012) Microenvironment of alginate-based microcapsules for cell culture and tissue engineering. J Biosci Bioeng 114:1–8 [DOI] [PubMed] [Google Scholar]
- 14.Calafiore R, Basta G (2014) Clinical application of microencapsulated islets: actual prospectives on progress and challenges. Adv Drug Deliv Rev 67–68:84–92 [DOI] [PubMed] [Google Scholar]
- 15.Ngoc PK, Phuc PV, Nhung TH, Thuy DT, Nguyet NTM (2011) Improving the efficacy of type 1 diabetes therapy by transplantation of immunoisolated insulin-producing cells. Hum Cell 24:86–95 [DOI] [PubMed] [Google Scholar]
- 16.Steele JAM, Hallé JP, Poncelet D, Neufeld RJ (2014) Therapeutic cell encapsulation techniques and applications in diabetes. Adv Drug Deliv Rev 67–68:74–83 [DOI] [PubMed] [Google Scholar]
- 17.Antosiak-Iwańska M, Sitarek E, Sabat M, Godlewska E, Kinasiewicz J, Weryński A (2009) Isolation, banking, encapsulation and transplantation of different types of Langerhans islets. Pol Arch Med Wewn 119:311–317 [PubMed] [Google Scholar]
- 18.Mallett AG, Korbutt GS (2009) Alginate modification improves long-term survival and function of transplanted encapsulated islets. Tissue Eng Part A 15:1301–1309 [DOI] [PubMed] [Google Scholar]
- 19.Scharp DW, Marchetti P (2014) Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution. Adv Drug Deliv Rev 67–68:35–73 [DOI] [PubMed] [Google Scholar]
- 20.de Haan BJ, Rossi A, Faas MM, Smelt MJ, Sonvico F, Colombo P et al. (2011) Structural surface changes and inflammatory responses against alginate-based microcapsules after exposure to human peritoneal fluid. J Biomed Mater Res A 98:394–403 [DOI] [PubMed] [Google Scholar]
- 21.Murua A, Orive G, Hernández RM, Pedraz JL (2009) Xenogeneic transplantation of erythropoietin-secreting cells immobilized in microcapsules using transient immunosuppression. J Control Release 137:174–178 [DOI] [PubMed] [Google Scholar]
- 22.Orive G, Tam SK, Pedraz JL, Hallé J-P (2006) Biocompatibility of alginate-poly-L-lysine microcapsules for cell therapy. Biomaterials 27:3691–3700 [DOI] [PubMed] [Google Scholar]
- 23.Arifin DR, Kedziorek DA, Fu Y, Chan KWY, McMahon MT, Weiss CR et al. (2013) Microencapsulated cell tracking. NMR Biomed 26:850–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnett BP, Arepally A, Stuber M, Arifin DR, Kraitchman DL, Bulte JWM (2011) Synthesis of magnetic resonance-, X-ray- and ultrasound-visible alginate microcapsules for immunoisolation and noninvasive imaging of cellular therapeutics. Nat Protoc 6:1142–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnett BP, Kraitchman DL, Lauzon C, Magee C, Walczak P, Gilson WD et al. (2006) Radiopaque alginate microcapsules for X-ray visualization and immunoprotection of cellular therapeutics. Mol Pharm 3:531–538 [DOI] [PubMed] [Google Scholar]
- 26.Kedziorek DA, Hofmann LV, Fu Y, Gilson WD, Cosby KM, Kohl B et al. (2012) X-ray-visible microcapsules containing mesenchymal stem cells improve hind limb perfusion in a rabbit model of peripheral arterial disease. Stem Cells 30:1286–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kedziorek DA, Solaiyappan M, Walczak P, Ehtiati T, Fu Y, Bulte JWM et al. (2013) Using C-Arm X-ray imaging to guide local reporter probe delivery for tracking stem cell engraftment. Theranostics 3:916–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan KW, Liu G, Song X, Kim H, Yu T, Arifin DR et al. (2013) MRI-detectable pH nanosensors incorporated into hydrogels for in vivo sensing of transplanted-cell viability. Nat Mater 12:268–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnett BP, Arepally A, Karmarkar PV, Qian D, Gilson WD, Walczak P et al. (2007) Magnetic resonance-guided, real-time targeted delivery and imaging of magnetocapsules immunoprotecting pancreatic islet cells. Nat Med 13:986–991 [DOI] [PubMed] [Google Scholar]
- 30.Link TW, Woodrum D, Gilson WD, Pan L, Qian D, Kraitchman DL et al. (2011) MR-guided portal vein delivery and monitoring of magnetocapsules: assessment of physiologic effects on the liver. J Vasc Interv Radiol 22:1335–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Link TW, Arifin DR, Long CM, Walczak P, Muja N, Arepally A et al. (2012) Use of magnetocapsules for in vivo visualization and enhanced survival of xenogeneic HepG2 cell transplants. Cell Med 4:77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arifin DR, Manek S, Call E, Arepally A, Bulte JW (2012) Microcapsules with intrinsic barium radiopacity for immunoprotection and X-ray/CT imaging of pancreatic islet cells. Biomaterials 33:4681–4689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J, Arifin DR, Muja N, Kim T, Gilad AA, Kim H et al. (2011) Multifunctional capsule-in-capsules for immunoprotection and trimodal imaging. Angew Chem Int Ed Engl 50:2317–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arifin DR, Long CM, Gilad AA, Alric C, Roux S, Tillement O et al. (2011) Trimodal gadolinium-gold microcapsules containing pancreatic islet cells restore normoglycemia in diabetic mice and can be tracked by using US, CT, and positive-contrast MR imaging. Radiology 260:790–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnett BP, Ruiz-Cabello J, Hota P, Liddell R, Walczak P, Howland V et al. (2011) Fluorocapsules for improved function, immunoprotection, and visualization of cellular therapeutics with MR, US, and CT imaging. Radiology 258:182–191 [DOI] [PMC free article] [PubMed] [Google Scholar]


