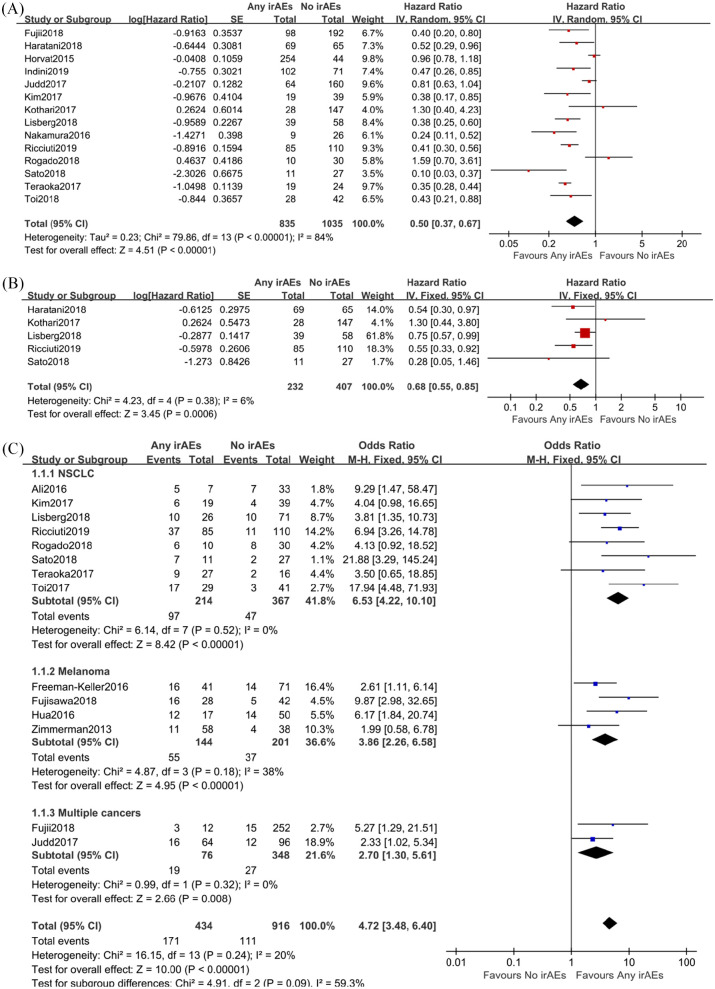
Figure 3.
Combined analysis of prognostic effect of any immune-related adverse events (irAEs) versus no irAEs on progression-free survival (PFS) and efficacy in cancer patients treated with immune checkpoint inhibitors. A, The association between irAEs and PFS; B, Assessing the association between irAEs and PFS based on landmark analysis results; C, Objective response rates in cancer patients with or without irAEs when treated with immune checkpoint inhibitors. Subgroup analysis was performed with regard to cancer types (non-small-cell lung carcinoma, melanoma, and other cancers).