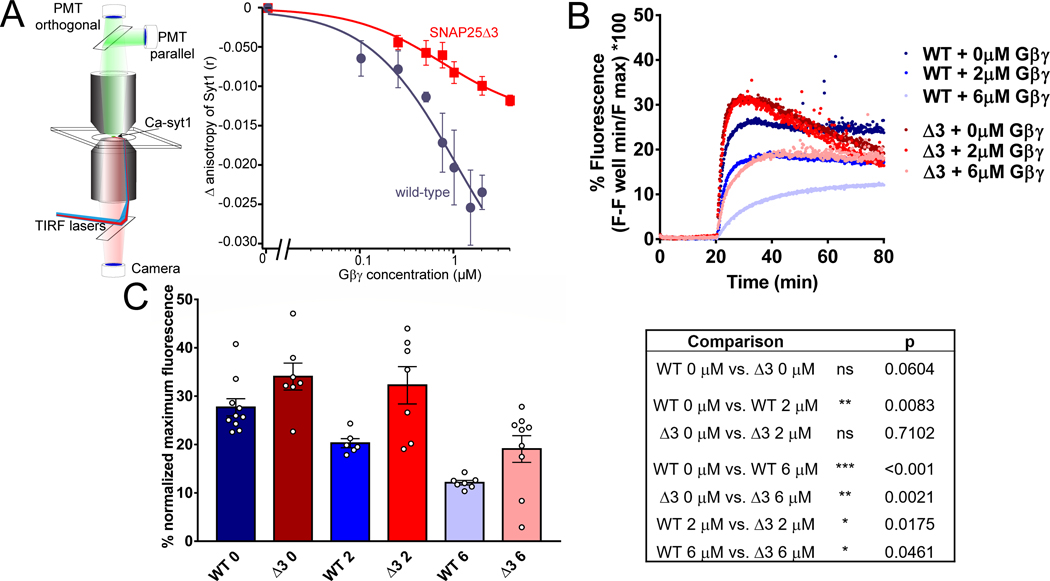
Figure 2. SNAP25Δ3 impairs Gβγ competition with synaptotagmin I and inhibition of calcium-synaptotagmin I-mediated liposome fusion.
A. Syt1 competition with Gβγ at t-SNARE complexes in lipid bilayers. Left panel: Schematic of the imaging system. A lipid bilayer consisting of 55% PC/15%PE/29%PS/1% DiD harboring t-SNARE complexes was fused to a glass coverslip and imaged using TIRF illumination from a 1.45 NA 60x lens through a laser TIRF illuminator. 1 μM Ca-AF-syt1 was applied over the bilayer (100 μM Ca2+). Graph shows Gβ1γ1 concentration dependence of the change in anisotropy produced by AF-syt I binding to WT (blue) or SNAP25Δ3 (red)-containing t-SNAREs embedded in the lipid membranes. The ability of Gβ1γ1 to displace AF-syt1 from SNAP25Δ3 t-SNAREs was reduced to 47±13% of its displacement of AF-syt1 from WT SNAP-25, as measured by change in anisotropy (n = 5 biological replicates per group, p = 0.019). AF-sytI displacement from WT t-SNAREs had an IC50 of 502 nM (95% CI: 150nM). B. Traces of lipid mixing experiments in which liposomes containing t-SNARE complexes made with SNAP25WT or SNAP25Δ3 were incubated with liposomes containing VAMP2 and a FRET pair of NBD-PE and rhodamine-PE in addition to 10μM sytI and Gβ1γ1. At t= 20 min, 1mM CaCl2 was added. C. Left panel: Bar graph of maximum fluorescence values: 2μM Gβγ significantly inhibits lipid mixing with liposomes containing t-SNAREs made with SNAP25WT (p=0.0083) but not SNAP25Δ3 (p = 0.71), while 6μM Gβγ inhibits significantly less in SNAP25Δ3 liposomes than SNAP25WT (p = 0.0461). Right panel: Table of significance values for lipid mixing experiments (Student’s two-tailed t-test). Experiments were repeated 6–8 times for 6–10 technical replicates.