Figure 1.
Purification of Budding Yeast Smc5/6 Holocomplex
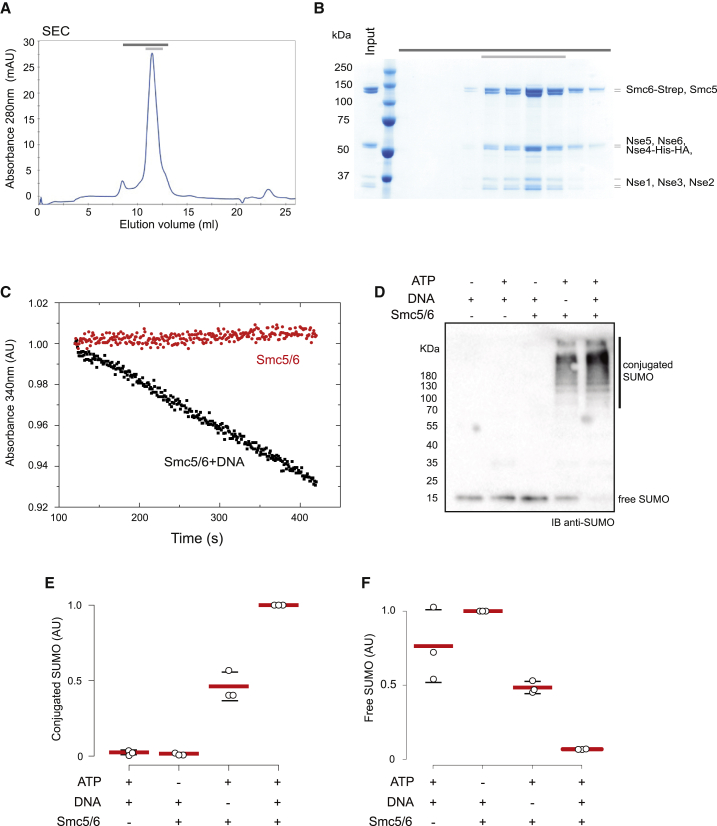
(A) Size-exclusion chromatogram (SEC) of wild-type Smc5/6 complexes.
(B) Analysis of peak fractions (dark gray bar) by SDS-PAGE and Coomassie staining. The pale gray bar indicates the pooled and concentrated fractions.
(C) Representative example of an ATPase activity assay of the Smc5/6 complex in the presence and absence of relaxed circular DNA. The linear fit of the absorbance data gives the ATPase rate consumption.
(D) Anti-SUMO western blot analysis of an in vitro SUMOylation reaction. Reactions were started by addition of 2 mM ATP and allowed to proceed for 15 min before being stopped by addition of SDS-PAGE loading buffer. Where indicated (+), Smc5/6 and DNA were added to 165 nM and 10 nM, respectively.
(E) Quantification of conjugated bands from three independent in vitro SUMOylation reactions. Mean (red lines) and standard deviation (black lines) values are shown. Circles represent the individual measurements for each of the experiments.
(F) Quantification of free SUMO bands from three independent in vitro SUMOylation reactions. Mean (red lines) and standard deviation (black lines) values are shown. Circles represent the individual measurements for each of the experiments.
See Table S1 for further characterization of Smc5/6 purification. See Figure S1 for further characterization of Smc5/6 SUMOylation activity.