Figure 6.
Smc5/6-Dependent DNA Compaction Is Sensitive to High Ionic Strength
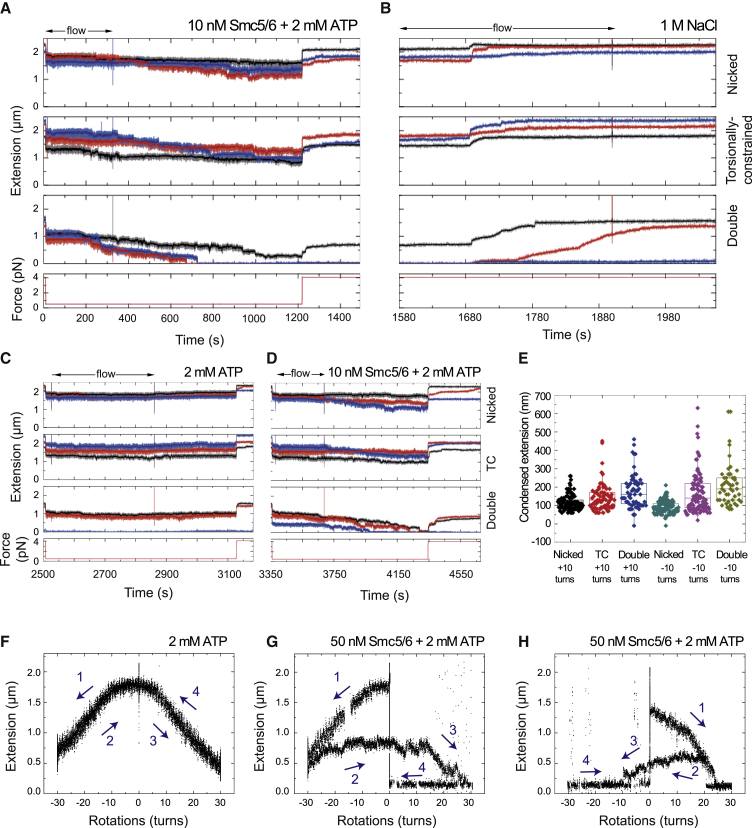
(A) Example of a sequential experiment where a sample containing 10 nM Smc5/6 with 2 mM ATP is first introduced at 0.5 pN while monitoring the DNA extension. Stepwise compaction (total or partial) of the tethers is observed. At the end of the experiment, the force increases to 4 pN, and the initial DNA extension is only partially recovered. Traces for individual molecules are shown (red, blue, and black).
(B) The fluidics cell is then washed with a high-salt (1 M NaCl) buffer, and the DNA molecules fully recover their initial extension at 4 pN. Traces for individual molecules are shown (red, blue, and black).
(C) After washing with buffer, a solution supplemented with 2 mM ATP but no protein is added, and the force is lowered to 0.5 pN. There is no apparent compaction under these conditions. Traces for individual molecules are shown (red, blue, and black).
(D) A fresh mixture of 10 nM Smc5/6 and 2 mM ATP is added, and clear condensation is observed again. Traces for individual molecules are shown (red, blue, and black).
(E) Condensed extensions when the initial topological state of the DNA is altered by +10 or −10 turns. We allow condensation to occur for a fixed time (90 s) at low force (0.5 pN) in the presence of 10 nM Smc5/6 and 2 mM ATP.
(F) Fully reversible rotation curve of a torsionally constrained DNA molecule at 0.5 pN in the presence of 2 mM ATP. First, 30 negative rotations are applied while keeping the force constant. After 120 s at −30 turns, the magnet was turned to 30 positive rotations for another 120 s and back to 0. When turns are released (magnets at 0 rotations), the initial end-to-end distance is fully recovered.
(G) Example of an irreversible rotation trace obtained with the same methodology as described in (F) but in the presence of 50 nM Smc5/6 and 2 mM ATP. In this case, the original end-to-end distance is not recovered when the magnet is rotated back to 0.
(H) Similar experiment as in (G), only here positive rotations are applied first.
Numbers and arrows in (F) and (G) represent the sequence of rotations. See Figures S6 and S7 for further characterization of Smc5/6 and condensin DNA compaction activities.