Figure 5. Changes in punctual stress are observed in an α-helix that functions in holoBirA dimerization and the loop C-terminal to it.
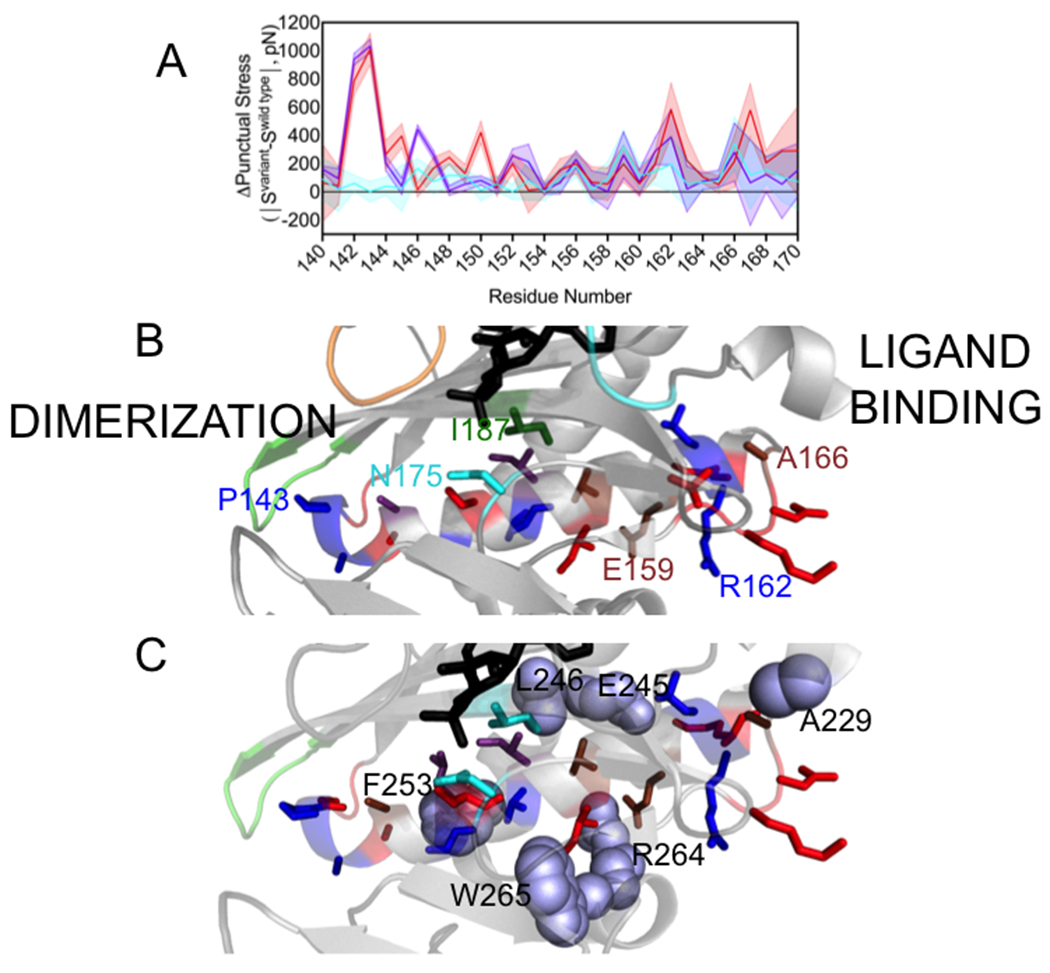
A. Punctual stress differences observed for residues in holoBirA helix 142-164 and the C-terminal segment 165-169: holoBirAM211A:cyan, holoBirAP143A:red, holoBirAP143A/M211A:purple. The shaded region represents the standard error in the punctual stress difference. B. Residue locations of punctual stress changes shown on the helix and loop with colors indicating variants in which punctual stress values differ from those found in holoBirAwt. Red: P143A, Blue: P143A and P143A/M211A, Brown: M211A, P143A and M211A/P143A, Cyan M211A (residue N175), Green: M211A and P143A (residue I187). Numbering is provided at select residue positions to orient the reader. C. Residues (lavender spheres) in holoBirAwt that form nonbonded interactions with dimerization helix and C-terminal extension residues and undergo changes punctual stress in the variants.
