Abstract
Background: Cannabis is increasingly used in Parkinson disease (PD), despite little information regarding benefits and risks.
Objectives: To investigate the safety and tolerability of a range of doses of cannabidiol (CBD), a nonintoxicating component of cannabis, and it's effect on common parkinsonian symptoms.
Methods: In this open-label study Coloradans with PD, substantial rest tremor, not using cannabis received plant-derived highly purified CBD (Epidiolex®; 100 mg/mL). CBD was titrated from 5 to 20–25 mg/kg/day and maintained for 10–15 days.
Results: Fifteen participants enrolled, two were screen failures. All 13 participants (10 male), mean (SD) age 68.15 (6.05), with 6.1 (4.0) years of PD, reported adverse events, including diarrhea (85%), somnolence (69%), fatigue (62%), weight gain (31%), dizziness (23%), abdominal pain (23%), and headache, weight loss, nausea, anorexia, and increased appetite (each 5%). Adverse events were mostly mild; none serious. Elevated liver enzymes, mostly a cholestatic pattern, occurred in five (38.5%) participants on 20–25 mg/kg/day, only one symptomatic. Three (23%) dropped out due to intolerance. Ten (eight male) that completed the study had improvement in total and motor Movement Disorder Society Unified Parkinson Disease Rating Scale scores of 7.70 (9.39, mean decrease 17.8%, p=0.012) and 6.10 (6.64, mean decrease 24.7%, p=0.004), respectively. Nighttime sleep and emotional/behavioral dyscontrol scores also improved significantly.
Conclusions: CBD, in the form of Epidiolex, may be efficacious in PD, but the relatively high dose used in this study was associated with liver enzyme elevations. Randomized controlled trials are needed to investigate various forms of cannabis in PD.
Keywords: cannabidiol, parkinsonism, Parkinson's disease, cannabis, adverse events
Introduction
Parkinson disease (PD) is a common neurodegenerative disorder characterized by motor symptoms of resting tremor, bradykinesia, rigidity, and impaired balance that often has nonmotor symptoms of cognitive dysfunction, anxiety, and psychosis. Since standard treatments may only partially relieve symptoms many patients are turning to complementary and alternative medications. In many states across the United States, cannabis use has been permitted medicinally and recreationally, so increasing numbers of PD patients are using formulations with a myriad of cannabinoid components. In an anonymous web-based survey, 47.8% of PD patients reported that cannabis reduced their use of prescription medication.1 Since the major component of the cannabis plant, Δ-9 tetrahydrocannabinol (THC), is known to sometimes cause psychosis,2–4 cognitive dysfunction,4–6 anxiety,7–9 and balance impairment,10,11 it may be especially harmful in PD. Conversely, cannabidiol (CBD), the next largest cannabinoid plant constituent, may have neuroprotective, anxiolytic, and antipsychotic effects,12 as well as relatively good tolerability; thus, it may have benefits in PD. The purpose of this study is to begin investigating the effects of cannabis in PD by focusing on the safety of a range of doses of a purified CBD formulation, Epidiolex®. In addition, the effect of CBD on common PD symptoms was studied.
Review of the literature shows that CBD is well tolerated at doses up to 1500 mg/day,13–15 or ∼13 mg/kg/day, and most definitively has a central nervous system effect, for example, reduction of seizures, at 25 mg/kg/day.16 However, the literature is sparse and inconclusive, and most studies used much lower doses, usually in combination with THC, and have shown a range of effects. A study in Huntington's disease using purified CBD at 10 mg/kg/day showed no effect.17 Thus, we designed this study to evaluate doses ranging from 5 to 25 mg/kg/day.
The U.S. governmental policies at the time this study was conducted required that human interventional cannabis research uses a study drug that is obtained from the National Institute on Drug Abuse (NIDA) or another Drug Enforcement Administration (DEA)-registered source. NIDA did not have a cannabis product with the desired dose of CBD that also had low enough THC content. Thus a highly purified form of CBD with minimal THC content was used for this study that was obtained from a DEA approved source.
Methods
Study participants
The trial was conducted at the University of Colorado Movement Disorders Center, an academic, tertiary referral center. Participants were eligible if they were 45–78 years old; lived in Colorado; met U.K. PD Society Brain Bank Clinical Diagnostic Criteria for idiopathic PD; and had resting tremor with at least an amplitude of ≥1 cm, that is, score of ≥2 on item 3.17 of the Movement Disorder Society Unified Parkinson Disease Rating Scale (MDS-UPDRS), in any limb while taking their usual PD medication, that is, while in the ON state. Participants had to agree not to operate a motor vehicle while taking the study drug, as required by the FDA. Key exclusion criteria included the following: cannabis detectable at the screening visit or THC detectable at the baseline visit; history of drug or alcohol dependence; use of dopamine antagonists within 180 days; and currently taking specified medications that are known to increase the risk of hepatotoxicity.
Written informed consent was obtained from all participants. This study is registered with ClinicalTrials.gov, NCT02818777, and approved by the Colorado Multiple Institutional Review Board. Study progress and safety were monitored by its Colorado Clinical & Translational Sciences Institute Drug Safety and Monitoring Board.
Study design and procedures
Participants in this open label, dose escalation study had a screening visit, a baseline visit within 3 weeks, a final assessment visit on their maximal dose, and a safety visit 2 weeks later. From the baseline visit, qualified study participants took a pharmaceutical formulation of highly purified CBD derived from Cannabis sativa L. plant in oral sesame oil solution (100 mg/mL, Epidiolex in the United States; GW Research Ltd., Cambridge, United Kingdom) with ≤0.15% THC twice daily. CBD was started at 5 mg/kg/day and was titrated by adjusting the dose every ≥4th day, by up to 5 mg/kg/day, until the maximum targeted (20–25 mg/kg/day) or tolerated dose was achieved. Participants maintained their maximal dose, as tolerated, for 10–15 days, and then stopped it the next day. A registered nurse or nurse practitioner interviewed participants using a standardized phone script at each dose regarding study drug effects and at 3 days after stopping CBD to check for signs of withdrawal. At study visits, efficacy assessments were conducted when the participants' PD medications were in optimal effect, that is, when participants were in their ON state. To monitor study drug compliance, participants filled out home diaries and study drug bottles were weighed. Participants maintained the same PD medications throughout the study period.
Dopaminergic medications were converted into levodopa equivalents (LE), according to the accepted formula18 (LE=immediate-release levodopa × 1 + controlled-release levodopa × 0.75 + pramipexole × 100 + ropinirole × 20 + rotigotine × 30 + selegiline × 10).
CBD analysis
Cannabinoid concentrations were measured in plasma samples collected during the screening visit, baseline visit (before the first dose was administered), at final assessment visit (3 h after the maximal dose was administered), and at the safety follow-up visit. A previously validated high-performance liquid chromatography atmospheric pressure chemical ionization mass spectrometry-based assay determined cannabinoid levels.19
Outcomes
The primary outcome was safety and tolerability of CBD and was measured in four ways: (1) the frequency and severity of adverse effects, using Common Terminology Criteria for Adverse Events terminology and grading, at each dose level; (2) vital signs, orthostatic blood pressures, physical examinations, electrocardiograms, and laboratory values (hematology, complete metabolic liver function tests, and urinalysis); (3) standardized assessment tools on relevant symptoms of PD; and (4) the proportion of participants that dropped out of the study due to study drug intolerance.
The prespecified major secondary outcome was the effect of CBD on severity and duration of tremor: the change from baseline to the maximal dose in the total of scores on items 3.17 and 3.18 in part III of the MDS-UPDRS in the ON state if the participant was taking anti-PD medication. Item 3.17 measures rest tremor amplitude, ranging from 0 (no tremor) to 4 (>10 cm in maximal amplitude) for each extremity and ranging from 0 (no tremor) to 4 (>3 cm in maximal amplitude) for lips/jaw. Item 3.18 measures constancy of rest tremor, ranging from 0 (no tremor) to 4 (present >75% of the examination). Other secondary outcomes were other motor signs and the common nonmotor symptoms of PD. The other motor signs were evaluated with the MDS-UPDRS parts II, III, and IV, as well as the Unified Dyskinesia Rating Scale (UDysRS). The effect of CBD on common nonmotor symptoms was measured using the following tools: MDS-UPDRS part I, Montreal Cognitive Assessment (MoCA), parts of Quality of Life in Neurological Disorders (Neurol-QOL) short forms for anxiety, depression, and emotional and behavioral dyscontrol, the Neuropsychiatric Inventory Questionnaire (NPI, © JL Cummings, 1994), Scales for Outcomes in PD-Sleep Scale (SCOPA-Sleep),20 Fatigue Severity Scale (FSS, © Lauren B. Knupps), the Patient Reported Outcome Measurement Information System (PROMIS) pain intensity and pain interference short forms, Impulsive-Compulsive disorders in Parkinson's disease Rating Scale (QUIP-RS), the Columbia-Suicide Severity Rating Scale (C-SSRS),21 International Restless Legs Syndrome Study Group Rating Scale (IRLS),22 and REM (rapid eye movement) sleep behavior disorder screening questionnaire (RBDSQ).23
Statistical analysis
Proportions presented for each adverse event type are the number of patients with any instance of the event divided by the total number of patients. Mean severity and standard deviation were calculated by averaging all the severity scores for each adverse event type across all the occurrences of the event within each patient and then taking the mean and standard deviation of the patient means, weighted by number of events. The same procedure was performed on any event type and by dose for any event type and several of the most common adverse event types. Generalized estimating equations (GEE) logistic regression models and Cochran-Mantel-Haenszel statistics were used to investigate the effect of dosage on the odds of adverse events.
Pre–Post changes in the motor and nonmotor scores were analyzed by performing permutation paired t-tests, with a univariate alpha=0.05 considered statistically significant. The Benjamini–Hochberg procedure was considered to control the false discovery rate (FDR) at alpha=0.05.
Findings
Participant characteristics
Between October 17, 2016 and June 19, 2017, 15 participants were enrolled, one was a screen failure due to electrocardiogram changes, one withdrew consent before starting treatment, thus 13 started study drug. Safety data are presented on these 13 participants. Three dropped out due to treatment related adverse events, thus 10 completed the study. Efficacy data are presented on these 10 participants. Note that these 10 participants are also in the Safety Group. Baseline characteristics of the 13 participants that took at least 1 dose of study drug, the Safety Group, and of the 10 participants, the Efficacy Group, that finished the study are described in Table 1.
Table 1.
Characteristics of the Participants at Baseline
| Safety analysis group (n=13) | Efficacy analysis groupa (n=10) | |
|---|---|---|
| Age, years, mean (SD) | 68.1 (6.05) | 68.7 (6.65) |
| Male, n (%) | 10 (77) | 8 (80) |
| Total MDS-UPDRS score, mean (SD) | 39.2 (13.3) | 43.2 (12.2) |
| Motor MDS-UPDRS score, mean (SD) | 22.9 (9.3) | 24.7 (8.9) |
| Disease duration, years, mean (SD) | 6.1 (4.0) | 6.3 (4.5) |
| H&Y, mean (SD) | 1.73 (0.56) | 1.75 (0.59) |
| MoCA, mean (SD) | 28.2 (1.6) | 27.9 (1.6) |
| Levodopa daily dose equivalent,b mean (SD) | 398.3 (331.0) | 443.8 (349.0) |
The 10 participants in the Efficacy Analysis Group are a subgroup of the 13 in the Safety Analysis Group. These are the 10 participants that completed the study.
Levodopa Daily Dose Equivalent (LE)=immediate release levodopa × 1 + controlled release levodopa × 0.75 + Pramipexole × 100 + Ropinirole × 20 + Rotigotine × 30 + Selegiline × 10.
H&Y, Hoehn and Yahr scale; MDS-UPDRS, Movement Disorder Society Unified Parkinson Disease Rating Scale; MoCA, Montreal Cognitive Assessment.
Safety and tolerability
Due to adverse events experienced by the first five enrolled participants, the maximal targeted dose was reduced from 25 to 20 mg/kg/day. The mean maximum CBD dose was 19.4 (SD 5.2) mg/kg/day, that is, 1623.0 mg/day (range 552.5–3458.8 mg/day) in the safety analysis group and 20.3 (3.4) mg/kg/day in the efficacy analysis group, that is, 1731.4 mg/day (range 1014.0–3458.8 mg/day). The mean maximum volume of sesame oil taken per participant per day was 16 mL (range 5.5–34.5 mL) in the safety analysis group. The average length of time on study drug was 26.8 (8.0) days in safety group and 28.5 (3.4) days in efficacy group.
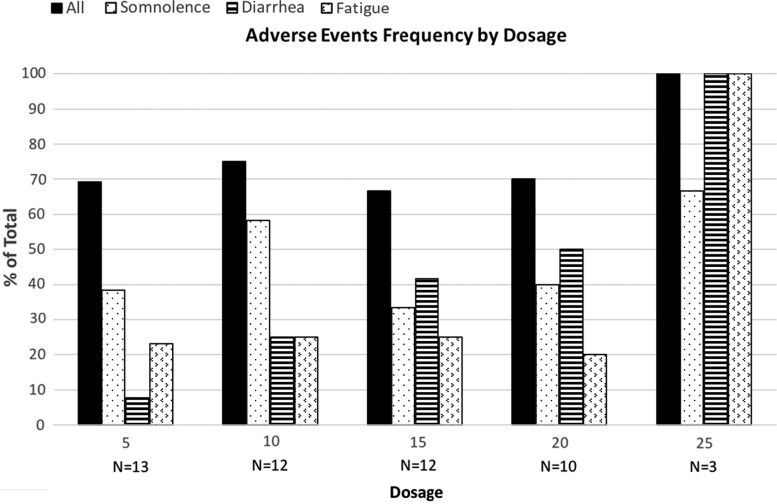
Adverse events, shown in Table 2, reported in all participants, were transient and mild (1.17±0.49) on average. The adverse events reported at each dose are shown in Figure 1. There were no serious adverse events and no withdrawal symptoms. The percentage of participants reporting diarrhea directly correlated with dosage, as shown in Figure 1. A GEE logistic regression model found that each 5 mg increase in the dosage increased the odds of diarrhea by an estimated factor of 2.32 (95% CI: 1.46–3.69, p=0.01).
Table 2.
Adverse Effects Reported in Safety Group Analysis (n=13)
| Adverse effectsa | Frequency, n (%) | Severity, mean (SD) |
|---|---|---|
| Any | 13 (100) | 1.17 (0.49) |
| Diarrhea | 11 (84.6) | 1.24 (0.68) |
| Somnolence | 9 (69.2) | 1.10 (0.31) |
| Fatigue | 8 (61.5) | 1.17 (0.22) |
| Weight gain | 4 (30.8) | 1.17 (0.33) |
| Abdominal pain | 3 (23.1) | 1 (0) |
| Dizziness | 3 (23.1) | 1 (0) |
| Weight loss, nausea, anorexia, increased appetite, headacheb | 2 (15.4) | N=6 (46.2%) 1.29 (0.37) |
| Vomiting, flatulence, gastroesophageal reflux disease, allergic reaction, spasm, fever, weaknessb | 1 (7.7) | N=6 (46.2%) 1.13 (0.27) |
Adverse effects terminology and severity is as per the Common Terminology Criteria for Adverse Events. Regarding severity, 1=mild, 2=moderate, 3=severe.
Individually each of the miscellaneous conditions occurs in only one or two patients, but when pooled for calculating the severity, 6 patients experience at least one.
FIG. 1.
Adverse event frequency by dosage.
There were no clinically significant adverse changes in other outcome safety assessments, except for increases in liver enzymes, especially alkaline phosphatase. Liver enzymes were measured at baseline and not again until the final assessment visit when participants were on their highest tolerated or the targeted dose. Elevations, shown in Table 3, occurred in five (38.5%) participants, one symptomatic and four asymptomatic, all on 20–25 mg/kg/day. The symptomatic participant, ID 02, developed moderate anorexia, diarrhea, somnolence, mild abdominal pain, dizziness, fatigue, fever, headache, and weight loss; had a cholestatic pattern of liver enzyme changes; and his liver ultrasound was normal. All symptoms and laboratory changes resolved after discontinuation of study drug.
Table 3.
Liver Function Test, Final Dose, and Medications
| ID | Age | Sex | ALT (normal 7–52 U/L) BL, final, SF | AST (normal 12–39 U/L) BL, final, SF | GGT (normal 9–64 U/L) BL, final, SF | ALP (normal 39–117 U/L) BL, final, SF, FU | T. Bili (normal 0.1–1.3 mg/dL) BL, final, SF | Final dose, mg/kg/day | Medications known to cause ANY hepatotoxicitya | Other medications |
|---|---|---|---|---|---|---|---|---|---|---|
| 02b | 70 | M | 11, 150, 49 | 16, 58, 23 | 9, 206, 116 | 99, 503, 247, 103 | 0.9, 1.4, 1.0 | 25 | Aspirin, celecoxib, rasagiline | None |
| 06b | 71 | M | 36, 64, 34 | 27, 46, 25 | 49, 101, 75 | 54, 81, 60 | 1.3, 1.8, 1.2 | 25 | Acetaminophen, fish oil, lisinopril, rasagiline, sildenafil, vitamin B6 | Biofreeze, glucosamine chondroitin, loperamide, magnesium, potassium citrate, vitamin B12, vitamin D3 |
| 08b | 70 | M | 12, 41, 9 | 17, 80, 29 | 29, 229, 229 | 12, 317, 182, 94 | 0.7, 0.9, 0.4 | 20 | Acetaminophen, aspirin, atorvastatin, doxazosin, esomeprazole, meloxicam | Carbidopa/levodopa, finasteride |
| 09b | 68 | M | 13, 29, 11 | 19, 22, 16 | 46, 129, 68 | 108, 137, 107 | 0.7, 0.7, 0.4 | 20 | Aspirin, ibuprofen, lisinopril, methylphenidate, ropinirole, selegiline, trazodone | Melatonin, vitamin D3 |
| 10b | 68 | M | 16, 20, 12 | 15, 30, 18 | 20, 162, 62 | 75, 134, 84 | 0.6, 0.6, 0.6 | 20 | Amantadine, aspirin, atorvastatin, citalopram, ibuprofen, omeprazole, Ropinirole | Carbidopa/levodopa, coQ10, folic acid, melatonin, vitamin C |
| 01 | 68 | F | 4, 8, N/A | 17, 20, N/A | N/A, 13, N/A | 62, 53, N/A | 0.4, 0.4, N/A | 17.5 | Escitalopram, fish oil, gabapentin, lansoprazole, lorazepam, ropinirole, prasterone | Carbidopa/levodopa, CoQ10, garlic, magnesium, melatonin, MiraLAX®, nystatin, senna, turmeric, vitamin B complex, vitamin D2 |
| 03 | 75 | M | 13, 14, N/A | 15, 19, N/A | 17, 16, N/A | 33, 31, N/A | 0.8, 1, N/A | 25 | Amlodipine, aspirin, hydrochlorothiazide, metoprolol, telmisartan | Docusate, calcium, etanercept, magnesium, vitamin B12 |
| 04 | 74 | M | 3, 8, N/A | 17, 25, N/A | 20, 24, N/A | 38, 38, N/A | 0.4, 0.5, N/A | 25 | Diclofenac, hydrochlorothiazide, docosahexaenoic acid, propranolol, selegiline | Carbidopa/levodopa, coQ 10, Heart Burn Relief®, multivitamin, stool softener. |
| 05 | 62 | M | 25, 25, N/A | 21, 29, N/A | 54, 51, N/A | 53, 49, N/A | 1, 0.8, N/A | 5 | Fish oil, ibuprofen (Advil®), meloxicam, olmesartan, rasagiline | Albuterol, coQ10, fluticasone, melatonin. |
| 07 | 74 | M | 3, 4, N/A | 19, 17, N/A | 13, 11, N/A | 54, 49, N/A | 0.5, 0.7, N/A | 20 | Aspirin, clonazepam, entacapone, loratadine, omeprazole, simvastatin, tramadol | Carbidopa/levodopa, Centrum®, ocuvite, omega 3, pramipexole, vitamin D3 |
| 11 | 58 | F | 20, 26, N/A | 18, 18, N/A | 15, 20, N/A | 57, 63, N/A | 0.5, 0.4, N/A | 20 | Estradiol, lisinopril, prasterone, progesterone, rasagiline | CoQ10, magnesium, melatonin, pramipexole |
| 13 | 73 | M | 25, 37, N/A | 26, 42, N/A | 24, 56, N/A | 52, 64, N/A | 0.7, 0.7, N/A | 12.5 | Acetaminophen, gabapentin, Ibuprofen, niacin/chromium | Carbidopa/levodopa, coQ10, ferrous sulfate |
| 14 | 56 | F | 4, 6, N/A | 19, 20, N/A | 11, 14, N/A | 80, 84, N/A | 0.4, 0.4, N/A | 20 | Conjugated estrogens, naproxen, nortriptyline, sumatriptan | Bone Nutrient®, carbidopa/levodopa, cellular vitality, diphenhydramine, essential oil, Food Nutrient® |
Hepatotoxicity is generally rare, unless the medication is in bold.
02 had symptomatic hepatitis. 06, 08, 09, and 10 had asymptomatic liver enzyme changes.
ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; BL, baseline visit; final, final visit; FU, follow-up visit, participants had ALP checked until it was in the normal range; GGT, gamma-glutamyl transferase; M, male; mg/dL, milligrams per deciliter; SF, safety follow-up visit 2 weeks after final visit; T. Bili, total bilirubin; U/L, units per liter.
Three participants (23%) stopped study drug due to intolerability, one due to rash at 5 mg/kg/day, one to abdominal pain and gas at 17.5 mg/kg/day, and one, described above, to fatigue, diarrhea, and elevated liver enzymes, that is, hepatitis, at 25 mg/kg/day.
Efficacy
The mean decreases in the total and motor MDS-UPDRS scores at the maximal dose compared with baseline were a 17.8% (p=0.012) and 24.7% (p=0.004) improvement, respectively. Two nonmotor assessments also showed significant improvement, the SCOPA-Sleep nighttime and the emotional and behavioral dyscontrol short form. At 2 weeks follow-up, the total MDS-UPDRS and nighttime sleep assessments remained improved; the other two assessments did not. Enrolled subjects had minimal levodopa induced dyskinesia, cognitive dysfunction, restless leg syndrome symptoms, REM sleep behavior disorder, and impulsivity at baseline, and these assessments did not change on study drug. Table 4 shows the data for all assessments.
Table 4.
Change in Motor and Nonmotor Scores Among Efficacy Analysis Group (n=10)
| Baseline, mean (SD) | Final, mean (SD) | Change from baseline to final, mean (SD) | pa | Follow-up, mean (SD) | Change from baseline to follow-up, mean (SD) | pa | |
|---|---|---|---|---|---|---|---|
| Rest Tremorb | 3.10 (2.23) | 2.70 (2.31) | −0.40 (1.26) | 0.471 | 4.20 (2.10) | 1.10 (2.56) | 0.109 |
| Total MDS-UPDRS | 43.20 (12.21) | 35.50 (14.31) | −7.70 (9.39) | 0.012a | 35.70 (14.97) | −7.50 (6.74) | 0.008a |
| Motor MDS-UPDRS | 24.70 (8.93) | 18.60 (9.66) | −6.10 (6.64) | 0.004a | 27.40 (10.51) | 2.70 (4.74) | 0.188 |
| H&Y | 1.75 (0.59) | 1.80 (0.89) | 0.05 (0.76) | 1.000 | 1.95 (0.685) | N/A | N/A |
| SCOPA-Sleep NS | 5.70 (2.95) | 2.90 (2.60) | −2.80 (3.91) | 0.040a | 3.30 (2.36) | −2.40 (3.66) | 0.023a |
| SCOPA-Sleep DS | 2.60 (2.27) | 2.20 (1.69) | −0.40 (1.65) | 0.575 | 2.10 (2.42) | −0.50 (1.84) | 0.453 |
| Emotional and behavioral dyscontrol SF | 44.39 (7.91) | 39.70 (6.75) | −4.69 (6.14) | 0.047a | 40.17 (7.08) | −4.22 (7.24) | 0.125 |
| NPI | 0.78 (1.20) | 0.75 (1.39) | −0.13 (1.55) | 1.000 | N/A | N/A | N/A |
| Anxiety SF | 46.24 (6.35) | 46.57 (7.27) | 0.33 (3.57) | 0.783 | 45.99 (7.05) | −0.25 (4.11) | 0.945 |
| Depression SF | 43.09 (6.03) | 42.24 (7.04) | −0.85 (3.13) | 0.440 | 41.1 (5.85) | −1.99 (4.55) | 0.500 |
| Fatigue Severity Scale | 28.50 (15.54) | 28.00 (14.49) | −0.50 (11.46) | 0.907 | 27.4 (14.91) | −1.10 (9.85) | 0.648 |
| Pain interference SF | 52.25 (8.63) | 49.93 (8.99) | −2.320 (6.008) | 0.253 | 49.94 (9.16) | −2.31 (6.99) | 0.438 |
| Pain intensity SF | 44.64 (7.36) | 42.78 (9.20) | −1.860 (4.711) | 0.311 | 42.96 (9.78) | −1.68 (5.20) | 0.469 |
Paired t-test, permutation distribution; p-values of less than 0.05 were considered to indicate statistical significance.
Rest tremor score was the prespecified major secondary outcome: total of the severity (MDS-UPDRS item 3.17) and duration (MDS-UPDRS item 3.18) of rest tremor.
NPI, Neuropsychiatric Inventory Questionnaire; SCOPA-Sleep DS, Scales for Outcomes in Parkinson's Disease-Sleep, Daytime Sleep; SCOPA-Sleep NS, Scales for Outcomes in Parkinson's Disease-Sleep, Nighttime Sleep; SD, standard deviation; SF, Short Form.
Applying the Benjamini–Hochberg procedure to control the FDR at alpha=0.05 found none of the tests to be statistically significant. The small sample size severely limits power for multiple testing adjustment.
CBD plasma levels
Three hours after the final dose was administered, the participant on 12.5 mg/kg/day had a CBD plasma level of 181 ng/mL, while those on 20 mg/kg/day, n=7, showed plasma levels of 376±78 ng/mL (mean±SEM) and those on 25 mg/kg/day, n=2, showed plasma levels of 340±4 ng/mL. Fourteen days after discontinuation of CBD, the values were 13, 24±5, and 39±14 ng/mL for the 12.5, 20, and 25 mg/kg/day treatment groups, respectively.
Discussion
This is the first study of the effects of relatively high dose, ∼20 mg/kg/day, purified CBD in humans with PD. The purpose of the study was to determine tolerability of a range of doses in the PD population and explore efficacy. The study found that relatively high dose CBD, ∼1600 mg/day, with <0.15% THC, that is, Epidiolex, taken orally, is associated with mild adverse effects, especially somnolence, fatigue, and diarrhea, and perhaps hepatotoxicity in persons with PD. In this dose ranging study somnolence occurred early in dose titration, but improved, diarrhea was common and increased with higher doses, and the 25 mg/kg/day dose was poorly tolerated. These adverse effects were similar to those reported in prior studies in pediatric epilepsy at this dose.16,24 However, they were more frequent in our population, perhaps due to different characteristics of the PD population or the smaller sample size in the present study. The only dose-related adverse event was diarrhea, which could be related to sesame oil. In three controlled trials conducted by GW Pharmaceuticals, manufacturer of Epidiolex, diarrhea was reported in 9% of participants on placebo, 9% on 10 mg/kg/day, and 20% on 20 mg/kg/day.a This suggests that the diarrhea is related to the CBD rather than sesame oil.
In this study five participants (38%) had transient elevated liver enzymes, one symptomatic and four asymptomatic, resolving after discontinuation of the study drug. In this study, the pattern of enzyme changes, particularly in the symptomatic patient, was consistent with a cholestatic rather than hepatocellular process. The drug may have caused idiopathic or bland cholestasis and, much less likely, granulomatous hepatitis or vanishing bile duct syndrome, since all liver tests normalized. Liver biopsy was not performed. None of these participants had significant elevated bilirubin or internalized normalized ratio, suggesting no change in liver function.
While liver enzyme elevations in a hepatocellular pattern have been reported with Epidiolex16 on doses similar to those taken by the present study participants, the cholestatic pattern has not. Single or multiple factors, such as older age, having PD, concomitant medications, and relatively high CBD dosage, may contribute to this. To date Epidiolex has been used primarily in pediatric populations. Perhaps older age and/or pathologic mechanisms that underlie PD, for example, mitochondrial dysfunction, are relevant factors. Regarding concomitant medications, most of the participants in prior epilepsy studies on Epidiolex were also on valproate; and while none in this PD study was, they were taking concomitant medications with potential hepatotoxicity, as shown in Table 3. Generally medications specific for PD have little potential for hepatotoxicity, with the exception of tolcapone, which none of the study participants was taking. There was no apparent diffence in the overall hepatotoxic potential of concomitant medications being taken by those that did and did not develop liver enzyme changes. Table 3 also shows the final CBD dose of participants, and again there was no apparent difference between those that did and did not have liver enzyme changes. Thus, it is unlikely that the liver enzyme elevations were solely due to hepatotoxicity of concomitant medications or CBD dose. Regarding dose, in this study liver enzymes were tested at the final, that is, highest dose, and not at lower doses, so the dose at which liver enzymes started to change is unknown. Perhaps persons with PD would have better tolerability of lower doses. Taken in whole, it is likely that multiple factors contributed to the liver enzyme changes that occurred in this study, with older age and relatively high CBD dosage being particularly relevant.
This was an open label study so no conclusions can be drawn regarding efficacy. However, assessments were done to check for hints of efficacy to inform future studies. The results suggest that CBD has a beneficial effect on total and motor MDS-UPDRS scores, nighttime sleep, and emotional and behavioral dyscontrol. Interestingly, the p-values on these assessments were still significantly or close to significantly improved 2 weeks after stopping CBD, compared to baseline. It is possible that CBD was still having some effect, since participants still had low plasma levels at that time.
A review of the literature of clinical studies of cannabinoids in PD shows that four randomized, blinded controlled studies have been reported, one Class 125 (according to the American Academy of Neurology Classification of Evidence for Rating of a Therapeutic Article, © 2014 American Academy of Neurology, AAN.com/guidelines), one Class 2,26 and two Class 3.27,28 Other studies were open label,29–31 case reports,32,33 or surveys.34–36 These other studies mostly evaluated cannabis; one included CBD,25 and three studied 99% pure CBD.26,29,32
The presented study is the first study of the effects of relatively high dose, ∼20 mg/kg/day, purified CBD in humans with PD. There were three prior studies of purified oral CBD, with reports of effects of lower doses. A double-blind study in 21 participants using ∼4.5 mg/kg/day found no change in UPDRS and other outcomes, except improved scores on the PD Questionnaire-39, which assessed functioning and well-being.26 An open label study in six patients found an improvement in total UPDRS scores and psychotic symptoms on ∼5 mg/kg/day of pure oral CBD.29 The third study was open label and reported that four participants taking ∼1–4 mg/kg/day had improvement in REM sleep behavior disorder, that is, reduction in acting out dreams.32 These three studies reported no adverse events. Other studies in PD examined the use of cannabis: two measuring the acute effects of smoked cannabis, total n=42, reported significantly improved motor UPDRS scores and reduced pain,30,31 another administered a medium dose of oral THC (0.25 mg/kg/day) and very low dose of CBD (0.125 mg/kg/day), n=17, and showed no significant change on all measured outcomes.25 Adverse effects of cannabis included somnolence, dizziness, decreased concentration, palpitations, and altered taste. Accumulating literature suggests that CBD may reduce psychosis,29,37 which is a frequent debilitating symptom in PD.
Most previous pre-clinical studies using animal models of PD have focused on cannabinoid receptor 1 (CB1) agonists and antagonists, rather than pure CBD. The studies show evidence of therapeutic effects, improving motor symptoms, and levodopa induced involuntary movements, that is, dyskinesia. However, these effects were found with both CB1 agonists and antagonists. Furthermore, there is a dose dependent effect: low doses of CB1 antagonists have been reported to improve motor function and dyskinesia more consistently than CB1 agonists, while high doses of both CB1 agonists and antagonists have no effects or impair motor function.38–47 Regarding CBD, a study using the 6-hydroxydopamine rat model of PD found that CBD, and also THC, attenuated neurodegeneration from the toxin, perhaps through antioxidant or anti-inflammatory mechanisms.48 Another study with the same toxin inducing PD, but in mice, studied pain thresholds and suggested that CBD modulated analgesic effects by increasing anandamide, a major endocannabinoid, levels and acting on CB1 and the transient receptor potential vanilloid receptor 1 (TRPV1) and that lower doses are more effective than intermediate doses.49 Evidence from pre-clinical studies to date suggests that there is more to learn about the effects of different types of cannabinoids on the motor signs of PD.38–47,50–54
How CBD influences PD is unclear, as its effect in humans is complex. CBD has low affinity for the endogenous cannabinoid receptors, but it can upregulate the levels of anandamide, by inactivation of fatty acid amide hydrolase, which metabolizes anandamide. Equally important, CBD interacts with many noncannabinoid signaling systems, and these functions may vary depending on its concentration.55 Activation of the serotonin 5-HT1A receptor may underlie improvement of motor signs.56 Other theories involve actions at G-protein coupled receptor 55,57,58 TRPV1,59,60 and GRP6 receptors. For example, the G-coupled protein receptor GPR6 is highly expressed in the basal ganglia. Depletion of GPR6 causes an increase of dopamine. By acting as an inverse agonist at the GPR6 receptor, CBD boosts dopamine levels in pre-clinical studies.61 A neuroprotective effect has been proposed due to studies showing anti-oxidant activity, for example, upregulating superoxide dismutase mRNA levels in the substantia nigra,62–65 and through anti-inflammatory activity, for example, activating peroxisome proliferator-activated receptor gamma.62,66–69
The plasma concentrations of CBD in our participants were consistent with prior pharmacokinetic studies of Epidiolex. Taylor et al.70 showed in a multiple ascending dose study of Epidiolex at 750 mg and 1500 mg twice daily (21.4 and 42.8 mg/kg/day for a 70 kg person) a tmax of ∼3–5 h after administration, depending on fasting versus nonfasting state. The Cmax was also dependent on the fasting state: 335 ng/mL when subjects were fasting versus 1628 ng/mL when subjects received a high-fat breakfast. In our study CBD plasma peak concentrations 3 h after the last administration of study drug of the treatment period (10–15 days) were 376±78 ng/mL (mean±SEM) for the 20 mg/kg/day (n=7). This is consistent with the Epidiolex PK study70 where 290 ng/mL (in the morning, fasting) and 732 ng/mL (in the afternoon, not fasting) were reported in the 750 mg (∼21 mg/kg/day) twice daily group. However, relatively high interindividual variabilities were observed in our study, likely because we did not control for food intake.
This study has some limitations. First, conclusions about adverse effects, but especially efficacy, are limited by the absence of a placebo arm. Also rater bias can occur in an open label design, both with regard to noting adverse effects, as well as rating scale assessments. Note, however, that the data that showed improved sleep and emotional and behavior dyscontrol were collected from participant questionnaires, rather than a rater. Furthermore, the number of participants was small and those included had minimal dyskinesia, cognitive dysfunction, restless leg syndrome, REM sleep behavior disorder, and impulse control disorder. These important problems may be altered by CBD treatment, but they were not addressed in this study. Furthermore, efficacy did not achieve significance when adjusted for multiple testing.
There was not a significant change in the major secondary outcome, tremor. Tremor was chosen because of the author's clinical experience: persons with PD in clinic frequently reported reduction in tremor with CBD, but rarely reported changes in other motor symptoms. Participants had to have a tremor amplitude in the ON state of at least 2 cm to be in the study, but tremor is variable from time to time and thus may be a less reliable motor symptom to measure. Quantifying tremor at home with a device may yield a more accurate measurement.
Further study of the effects of cannabinoids in PD is greatly needed, since presently these persons are trying products with various cannabinoid compositions despite little data regarding safety and efficacy. The usual dose of CBD in dispensary products is quite variable, for example, 2–200 mg/day, is much lower than that used in this study, and the CBD is often combined with clinically relevant doses of THC. Besides dosage, routes of administration might alter outcomes, for example, smoking or vaping would likely cause more immediate intense and shorter lasting effects. As increasing numbers of persons with PD visit cannabis dispensaries, they are presented with a wide range of choices. Randomized controlled studies are needed to confirm this study's findings and to investigate lower doses of CBD, CBD in combination with THC at varying compositions, and the effects of routes of administration.
Acknowledgments
The authors are grateful to Emil Diguilio, Sarah Fischer, and Candace Ellman for their assistance in preparation of this article, to Nicole Gendelman and the University of Colorado Department of Neurology Core Clinical Trials Team for their help with all aspects of conducting this study, and especially thank the patients that participated in this study.
Abbreviations Used
- CB1
cannabinoid receptor 1
- CBD
cannabidiol
- C-SSRS
Columbia-Suicide Severity Rating Scale
- DEA
Drug Enforcement Administration
- FDR
false discovery rate
- FSS
Fatigue Severity Scale
- H&Y
Hoehn and Yahr scale
- IRLS
International Restless Legs Syndrome Study Group Rating Scale
- LE
levodopa equivalents
- MDS-UPDRS
Movement Disorder Society Unified Parkinson Disease Rating Scale
- MoCA
Montreal Cognitive Assessment
- Neurol-QOL
Quality of Life in Neurological Disorders
- NIDA
National Institute on Drug Abuse
- NPI
Neuropsychiatric Inventory Questionnaire
- PD
Parkinson disease
- PROMIS
Patient Reported Outcome Measurement Information System
- QUIP-RS
Impulsive-Compulsive disorders in Parkinson's disease Rating Scale
- RBDSQ
REM sleep behavior disorder screening questionnaire
- REM
rapid eye movement
- SCOPA-Sleep
Scales for Outcomes in PD-Sleep Scale
- THC
Δ-9 tetrahydrocannabinol
- TRPV1
transient receptor potential vanilloid receptor 1
- UDysRS
Unified Dyskinesia Rating Scale
Author Disclosure Statement
No competing financial interests exist.
Funding Information
The study is funded by Colorado Department of Public Health and Environment. The study drug was provided by GW Research Ltd., Cambridge, United Kingdom. The study is supported by NIH/NCATS Colorado CTSA grant number UL1 TR001082-05. Contents are the authors' sole responsibility and do not necessarily represent official NIH views. Certain results from this study were generated using the “Questionnaire for Impulse Control Disorders in Parkinson's Disease (QUIP-RS),” licensed from the University of Pennsylvania.
Cite this article as: Leehey MA, Liu Y, Hart F, Epstein C, Cook M, Sillau S, Klawitter J, Newman H, Sempio C, Forman L, Seeberger L, Klepitskaya O, Baud Z, Bainbridge J (2020) Safety and tolerability of cannabidiol in Parkinson disease: an open label, dose-escalation study, Cannabis and Cannabinoid Research 5:4, 326–336, DOI: 10.1089/can.2019.0068.
Epidiolex [package insert]. Carlsbad, CA: Greenwich Biosciences, Inc; 2020. Available at: https://www.epidiolex.com/sites/default/files/EPIDIOLEX_Full_Prescribing_Information.pdf_ga=2.42410341.1764361511.1577819316-2124301189.1577819316
References
- 1. Kindred JH, Li K, Ketelhut NB, et al. . Cannabis use in people with Parkinson's disease and multiple sclerosis: a web-based investigation. Complement Ther Med. 2017;33:99–104 [DOI] [PubMed] [Google Scholar]
- 2. Bhattacharyya S, Crippa JA, Allen P, et al. . Induction of psychosis by Delta9-tetrahydrocannabinol reflects modulation of prefrontal and striatal function during attentional salience processing. Arch Gen Psychiatry. 2012;69:27–36 [DOI] [PubMed] [Google Scholar]
- 3. Murray RM, Englund A, Abi-Dargham A, et al. . Cannabis-associated psychosis: neural substrate and clinical impact. Neuropharmacology. 2017;124:89–104 [DOI] [PubMed] [Google Scholar]
- 4. Morrison PD, Zois V, McKeown DA, et al. . The acute effects of synthetic intravenous Delta9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychol Med. 2009;39:1607–1616 [DOI] [PubMed] [Google Scholar]
- 5. Englund A, Atakan Z, Kralj A, et al. . The effect of five day dosing with THCV on THC-induced cognitive, psychological and physiological effects in healthy male human volunteers: a placebo-controlled, double-blind, crossover pilot trial. J Psychopharmacol. 2016;30:140–151 [DOI] [PubMed] [Google Scholar]
- 6. Curran HV, Brignell C, Fletcher S, et al. . Cognitive and subjective dose-response effects of acute oral Delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology. 2002;164:61–70 [DOI] [PubMed] [Google Scholar]
- 7. Boggs DL, Nguyen JD, Morgenson D, et al. . Clinical and pre-clinical evidence for functional interactions of cannabidiol and Delta9-tetrahydrocannabinol. Neuropsychopharmacology. 2017;75:157–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. D'Souza DC, Perry E, MacDougall L, et al. . The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572 [DOI] [PubMed] [Google Scholar]
- 9. Karniol IG, Shirakawa I, Kasinski N, et al. . Cannabidiol interferes with the effects of delta 9-tetrahydrocannabinol in man. Eur J Pharmacol. 1974;28:172–177 [DOI] [PubMed] [Google Scholar]
- 10. Newmeyer MN, Swortwood MJ, Taylor ME, et al. . Evaluation of divided attention psychophysical task performance and effects on pupil sizes following smoked, vaporized and oral cannabis administration. J Appl Toxicol. 2017;37:922–932 [DOI] [PubMed] [Google Scholar]
- 11. Bosker WM, Theunissen EL, Conen S, et al. . A placebo-controlled study to assess Standardized Field Sobriety Tests performance during alcohol and cannabis intoxication in heavy cannabis users and accuracy of point of collection testing devices for detecting THC in oral fluid. Psychopharmacology. 2012;223:439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Campos AC, Fogaca MV, Sonego AB, et al. . Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res. 2016;112:119–127 [DOI] [PubMed] [Google Scholar]
- 13. Bergamaschi MM, Queiroz RH, Zuardi AW, et al. . Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf. 2011;6:237–249 [DOI] [PubMed] [Google Scholar]
- 14. Devinsky O, Patel AD, Cross JH, et al. . Effect of cannabidiol on drop seizures in the Lennox-Gastaut Syndrome. N Engl J Med. 2018;378:1888–1897 [DOI] [PubMed] [Google Scholar]
- 15. Thiele EA, Marsh ED, French JA, et al. . Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391:1085–1096 [DOI] [PubMed] [Google Scholar]
- 16. Devinsky O, Cross JH, Laux L, et al. . Trial of cannabidiol for drug-resistant seizures in the Dravet Syndrome. N Engl J Med. 2017;376:2011–2020 [DOI] [PubMed] [Google Scholar]
- 17. Consroe P, Laguna J, Allender J, et al. . Controlled clinical trial of cannabidiol in Huntington's disease. Pharmacol Biochem Behav. 1991;40:701–708 [DOI] [PubMed] [Google Scholar]
- 18. Tomlinson CL, Stowe R, Patel S, et al. . Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649–2653 [DOI] [PubMed] [Google Scholar]
- 19. Klawitter J, Sempio C, Morlein S, et al. . An atmospheric pressure chemical ionization MS/MS assay using online extraction for the analysis of 11 cannabinoids and metabolites in human plasma and urine. Ther Drug Monit. 2017;39:556–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marinus J, Visser M, van Hilten JJ, et al. . Assessment of sleep and sleepiness in Parkinson disease. Sleep. 2003;26:1049–1054 [DOI] [PubMed] [Google Scholar]
- 21. Posner K, Brown GK, Stanley B, et al. . The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Walters AS, LeBrocq C, Dhar A, et al. . Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–132 [DOI] [PubMed] [Google Scholar]
- 23. Stiasny-Kolster K, Mayer G, Schafer S, et al. . The REM sleep behavior disorder screening questionnaire—a new diagnostic instrument. Mov Disord. 2007;22:2386–2393 [DOI] [PubMed] [Google Scholar]
- 24. Devinsky O, Marsh E, Friedman D, et al. . Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15:270–278 [DOI] [PubMed] [Google Scholar]
- 25. Carroll CB, Bain PG, Teare L, et al. . Cannabis for dyskinesia in Parkinson disease: a randomized double-blind crossover study. Neurology. 2004;63:1245–1250 [DOI] [PubMed] [Google Scholar]
- 26. Chagas MH, Zuardi AW, Tumas V, et al. . Effects of cannabidiol in the treatment of patients with Parkinson's disease: an exploratory double-blind trial. J Psychopharmacol. 2014;28:1088–1098 [DOI] [PubMed] [Google Scholar]
- 27. Sieradzan KA, Fox SH, Hill M, et al. . Cannabinoids reduce levodopa-induced dyskinesia in Parkinson's disease: a pilot study. Neurology. 2001;57:2108–2111 [DOI] [PubMed] [Google Scholar]
- 28. Mesnage V, Houeto JL, Bonnet AM, et al. . Neurokinin B, neurotensin, and cannabinoid receptor antagonists and Parkinson disease. Clin Neuropharmacol. 2004;27:108–110 [DOI] [PubMed] [Google Scholar]
- 29. Zuardi AW, Crippa JA, Hallak JE, et al. . Cannabidiol for the treatment of psychosis in Parkinson's disease. J Psychopharmacol. 2009;23:979–983 [DOI] [PubMed] [Google Scholar]
- 30. Lotan I, Treves TA, Roditi Y, et al. . Cannabis (medical marijuana) treatment for motor and non-motor symptoms of Parkinson disease: an open-label observational study. Clin Neuropharmacol. 2014;37:41–44 [DOI] [PubMed] [Google Scholar]
- 31. Shohet A, Khlebtovsky A, Roizen N, et al. . Effect of medical cannabis on thermal quantitative measurements of pain in patients with Parkinson's disease. Eur J Pain. 2017;21:486–493 [DOI] [PubMed] [Google Scholar]
- 32. Chagas MH, Eckeli AL, Zuardi AW, et al. . Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson's disease patients: a case series. J Clin Pharm Ther. 2014;39:564–566 [DOI] [PubMed] [Google Scholar]
- 33. Frankel JP, Hughes A, Lees AJ, et al. . Marijuana for parkinsonian tremor. J Neurol Neurosurg Psychiatry. 1990;53:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Finseth TA, Hedeman JL, Brown RP, 2nd, et al. Self-reported efficacy of cannabis and other complementary medicine modalities by Parkinson's disease patients in colorado. Evid Based Complement Alternat Med. 2015;2015:874849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Venderova K, Ruzicka E, Vorisek V, et al. . Survey on cannabis use in Parkinson's disease: subjective improvement of motor symptoms. Mov Disord. 2004;19:1102–1106 [DOI] [PubMed] [Google Scholar]
- 36. Balash Y, Bar-Lev Schleider L, Korczyn AD, et al. . Medical cannabis in Parkinson disease: real-life patients' experience. Clin Neuropharmacol. 2017;40:268–272 [DOI] [PubMed] [Google Scholar]
- 37. McGuire P, Robson P, Cubala WJ, et al. . Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175:225–231 [DOI] [PubMed] [Google Scholar]
- 38. Garcia C, Palomo-Garo C, Garcia-Arencibia M, et al. . Symptom-relieving and neuroprotective effects of the phytocannabinoid Delta(9)-THCV in animal models of Parkinson's disease. Br J Pharmacol. 2011;163:1495–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gutierrez-Valdez AL, Garcia-Ruiz R, Anaya-Martinez V, et al. . The combination of oral L-DOPA/rimonabant for effective dyskinesia treatment and cytological preservation in a rat model of Parkinson's disease and L-DOPA-induced dyskinesia. Behav Pharmacol. 2013;24:640–652 [DOI] [PubMed] [Google Scholar]
- 40. Gonzalez S, Scorticati C, Garcia-Arencibia M, et al. . Effects of rimonabant, a selective cannabinoid CB1 receptor antagonist, in a rat model of Parkinson's disease. Brain Res. 2006;1073–1074:209–219 [DOI] [PubMed] [Google Scholar]
- 41. van der Stelt M, Fox SH, Hill M, et al. . A role for endocannabinoids in the generation of parkinsonism and levodopa-induced dyskinesia in MPTP-lesioned non-human primate models of Parkinson's disease. FASEB J. 2005;19:1140–1142 [DOI] [PubMed] [Google Scholar]
- 42. Kelsey JE, Harris O, Cassin J. The CB(1) antagonist rimonabant is adjunctively therapeutic as well as monotherapeutic in an animal model of Parkinson's disease. Behav Brain Res. 2009;203:304–307 [DOI] [PubMed] [Google Scholar]
- 43. Walsh S, Gorman AM, Finn DP, et al. . The effects of cannabinoid drugs on abnormal involuntary movements in dyskinetic and non-dyskinetic 6-hydroxydopamine lesioned rats. Brain Res. 2010;1363:40–48 [DOI] [PubMed] [Google Scholar]
- 44. Cao X, Liang L, Hadcock JR, et al. . Blockade of cannabinoid type 1 receptors augments the antiparkinsonian action of levodopa without affecting dyskinesias in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated rhesus monkeys. J Pharmacol Exp Ther. 2007;323:318–326 [DOI] [PubMed] [Google Scholar]
- 45. Fernandez-Espejo E, Caraballo I, de Fonseca FR, et al. . Cannabinoid CB1 antagonists possess antiparkinsonian efficacy only in rats with very severe nigral lesion in experimental parkinsonism. Neurobiol Dis. 2005;18:591–601 [DOI] [PubMed] [Google Scholar]
- 46. Segovia G, Mora F, Crossman AR, et al. . Effects of CB1 cannabinoid receptor modulating compounds on the hyperkinesia induced by high-dose levodopa in the reserpine-treated rat model of Parkinson's disease. Mov Disord. 2003;18:138–149 [DOI] [PubMed] [Google Scholar]
- 47. Morgese MG, Cassano T, Cuomo V, et al. . Anti-dyskinetic effects of cannabinoids in a rat model of Parkinson's disease: role of CB(1) and TRPV1 receptors. Exp Neurol. 2007;208:110–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lastres-Becker I, Molina-Holgado F, Ramos JA, et al. . Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson's disease. Neurobiol Dis. 2005;19:96–107 [DOI] [PubMed] [Google Scholar]
- 49. Crivelaro do Nascimento G, Ferrari DP, Guimaraes FS, et al. . Cannabidiol increases the nociceptive threshold in a preclinical model of Parkinson's disease. Neuropharmacology. 2020;163:107808. [DOI] [PubMed] [Google Scholar]
- 50. Martinez A, Macheda T, Morgese MG, et al. . The cannabinoid agonist WIN55212–2 decreases L-DOPA-induced PKA activation and dyskinetic behavior in 6-OHDA-treated rats. Neurosci Res. 2012;72:236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gilgun-Sherki Y, Melamed E, Mechoulam R, et al. . The CB1 cannabinoid receptor agonist, HU-210, reduces levodopa-induced rotations in 6-hydroxydopamine-lesioned rats. Pharmacol Toxicol. 2003;93:66–70 [DOI] [PubMed] [Google Scholar]
- 52. Fox SH, Henry B, Hill M, et al. . Stimulation of cannabinoid receptors reduces levodopa-induced dyskinesia in the MPTP-lesioned nonhuman primate model of Parkinson's disease. Mov Disord. 2002;17:1180–1187 [DOI] [PubMed] [Google Scholar]
- 53. Meschler JP, Howlett AC, Madras BK. Cannabinoid receptor agonist and antagonist effects on motor function in normal and 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine (MPTP)-treated non-human primates. Psychopharmacology. 2001;156:79–85 [DOI] [PubMed] [Google Scholar]
- 54. Moss DE, McMaster SB, Rogers J. Tetrahydrocannabinol potentiates reserpine-induced hypokinesia. Pharmacol Biochem Behav. 1981;15:779–783 [DOI] [PubMed] [Google Scholar]
- 55. Devinsky O, Cilio MR, Cross H, et al. . Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55:791–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Peres FF, Lima AC, Hallak JEC, et al. . Cannabidiol as a promising strategy to treat and prevent movement disorders? Front Pharmacol. 2018;9:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sylantyev S, Jensen TP, Ross RA, et al. . Cannabinoid- and lysophosphatidylinositol-sensitive receptor GPR55 boosts neurotransmitter release at central synapses. Proc Natl Acad Sci U S A. 2013;110:5193–5198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bazelot MJN, Whalley BJ. Effects of cannabidiol on LPI-induced GPR55-mediated excitatory synaptic transmission at CA3-CA1 principal neuron synapses in healthy and epileptic rats. American Academy of Neurology Annual Meeting, Vancouver, BC, 2016 [Google Scholar]
- 59. Iannotti FA, Hill CL, Leo A, et al. . Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci. 2014;5:1131–1141 [DOI] [PubMed] [Google Scholar]
- 60. Saffarzadeh F, Eslamizade MJ, Mousavi SM, et al. . TRPV1 receptors augment basal synaptic transmission in CA1 and CA3 pyramidal neurons in epilepsy. Neuroscience. 2016;314:170–178 [DOI] [PubMed] [Google Scholar]
- 61. Laun AS, Song ZH. GPR3 and GPR6, novel molecular targets for cannabidiol. Biochem Biophys Res Commun. 2017;490:17–21 [DOI] [PubMed] [Google Scholar]
- 62. Fernandez-Ruiz J, Sagredo O, Pazos MR, et al. . Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid? Br J Clin Pharmacol. 2013;75:323–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fernandez-Ruiz J, Garcia C, Sagredo O, et al. . The endocannabinoid system as a target for the treatment of neuronal damage. Expert Opin Ther Targets. 2010;14:387–404 [DOI] [PubMed] [Google Scholar]
- 64. Hampson AJ, Grimaldi M, Axelrod J, et al. . Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A. 1998;95:8268–8273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Garcia-Arencibia M, Gonzalez S, de Lago E, et al. . Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson's disease: importance of antioxidant and cannabinoid receptor-independent properties. Brain Res. 2007;1134:162–170 [DOI] [PubMed] [Google Scholar]
- 66. O'Sullivan SE, Sun Y, Bennett AJ, et al. . Time-dependent vascular actions of cannabidiol in the rat aorta. Eur J Pharmacol. 2009;612:61–68 [DOI] [PubMed] [Google Scholar]
- 67. Esposito G, Scuderi C, Valenza M, et al. . Cannabidiol reduces Abeta-induced neuroinflammation and promotes hippocampal neurogenesis through PPARgamma involvement. PLoS One. 2011;6:e28668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dos-Santos-Pereira M, da-Silva CA, Guimaraes FS, et al. . Co-administration of cannabidiol and capsazepine reduces L-DOPA-induced dyskinesia in mice: possible mechanism of action. Neurobiol Dis. 2016;94:179–195 [DOI] [PubMed] [Google Scholar]
- 69. Barbiero JK, Santiago RM, Persike DS, et al. . Neuroprotective effects of peroxisome proliferator-activated receptor alpha and gamma agonists in model of parkinsonism induced by intranigral 1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine. Behav Brain Res. 2014;274:390–399 [DOI] [PubMed] [Google Scholar]
- 70. Taylor L, Gidal B, Blakey G, et al. . A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs. 2018;32:1053–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]