Abstract
Fistulas in head and neck cancer patients are a common and challenging issue. Despite their commonality, there is little consensus regarding optimal treatment strategies or in preventative measures that might be taken preoperatively. A general knowledge and understanding of what factors correlate with fistula formation can assist a surgeon in optimizing a patient for surgery, thus decreasing prevalence. In addition, surgical techniques can aid in both the prevention and treatment of fistulas once they form. This review details risk factors for fistula formation, the use of vascularized tissue as a preventative measure, conservative and nonconservative treatment of fistulas, and possible strategies to decrease the likelihood of their formation.
Keywords: fistula, head and neck cancer, regional flap, free flap, laryngectomy
Fistulas of the head and neck are a common and well-known complication of head and neck cancer that pose unique challenges for head and neck or reconstructive surgeon. Defined as an abnormal connection between two epithelialized spaces, fistulae generally begin as a collecting pocket of saliva from the pharynx, eventually fistulizing through the skin. They may form following radiotherapy, neck dissection, glossectomy, laryngectomy, or any other surgery which has the potential of causing a connection between the digestive tract and the surrounding tissues of the neck. With incidences as high as 15 to 27% following total laryngectomy, proper management of head and neck fistulas is crucial. 1 2 With sequelae of fistulas ranging from infection to free flap failure or even carotid blowout, prompt recognition also becomes an important aspect of preventing further complications or delays in the patient's recovery and treatment. Despite their common occurrence, method of treatment remains controversial and situation-dependent without uniform guidelines or recommendations.
Risk Factors for Fistula Formation
When patients can be identified as being at higher risk for fistula formation, they can be more closely monitored for signs and symptoms and subsequently be treated promptly. Prevention by optimization of certain risk factors would be ideal, and thus there is abundant literature attempting to find such correlations.
One systematic review which looked at pharyngocutaneous fistula formation following total laryngectomy found that a history of chronic obstructive pulmonary disease, a hemoglobin of less than 12.5 g/dL, or recent history of a blood transfusion all increased risk of fistula formation. 3 Treatment itself was also found to correlate with risk of fistula formation. In this same study, 24.6% of patients who underwent radiotherapy prior to total laryngectomy presented with a fistula, compared with 15.5% of patients who did not. A T3 or T4 larynx cancer was also found to increase risk of fistula formation by 4% as compared with T1 or T2 cancers. If a neck dissection was performed in conjunction with the laryngectomy, an added risk of 6% was observed. Concurrent chemotherapy at the time of surgery also correlates with increased risk. 4
In another study by Mattioli et al, which specifically analyzed patients who underwent a total laryngectomy, both preoperative malnutrition (determined by albumin and prealbumin levels) and diabetes were found to be correlated with fistula formation. 5 There is also evidence that both surgical site infections and methicillin-resistant staphylococcus aureus colonization are associated with fistula formation. 6 It has also been suggested that timing of salvage laryngectomy following previous radiation therapy also correlates with fistula formation. Basheeth et al found that salvage laryngectomy within 1 year of previous radiation therapy increased risk of fistula formation from 11 to 34%. 7 Another study found that the presence of postoperative hypothyroidism following salvage laryngectomy correlated with a fistula incidence of 47%, as opposed to 23% in euthyroid patients. 8 With both hypothyroidism and low albumin and prealbumin levels correlating with fistula formation risk, it would be prudent to evaluate these laboratories prior to surgery. As many patients undergo surgery relatively quickly after diagnosis, it is not always possible to completely alleviate these concerns preoperatively. However, a surgeon may consider encouraging higher caloric intake and high calorie nutritional supplements preoperatively as possible.
Fistula formation is certainly not limited to larynx cancers or even surgery as they can develop after any type of acquired wound. Fig. 1 demonstrates a patient with an extensive ulcer following chemoradiation for a T3 larynx cancer that ultimately led to a laryngocutaneous fistula with carotid blowout. Girkar et al explored risk factors for orocutaneous fistula formation following surgery for oral cancer. They found that patients who also underwent a bilateral neck dissection or developed a surgical site infection were more likely to develop a fistula compared with those who did not. 9
Fig. 1.
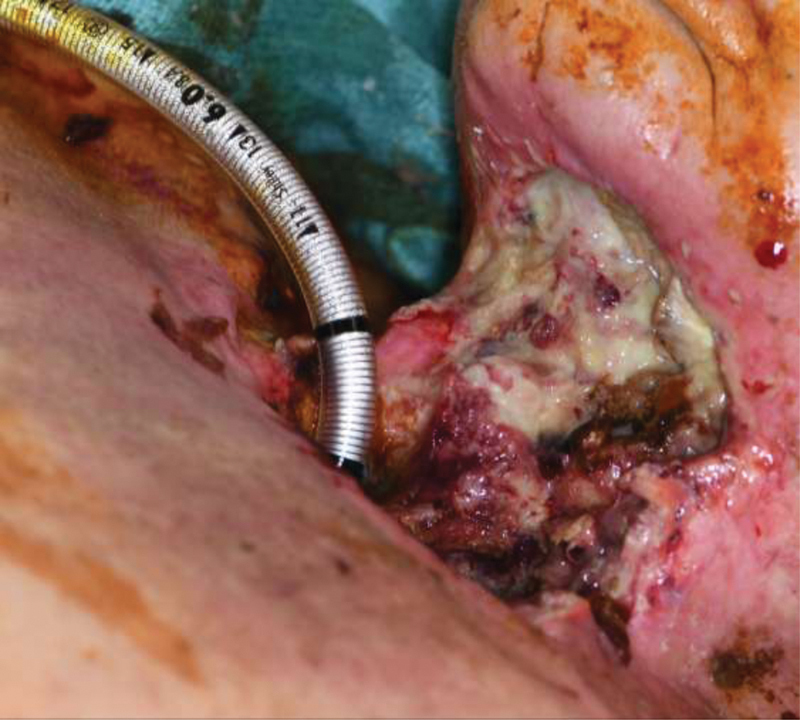
Patient presented 3 months following chemoradiation for T3 larynx cancer with extensive ulcer and laryngocutaneous fistula of anterior neck, left carotid atmospheric fistula with blowout. Intraoperatively, left common carotid artery was ligated.
Use of Vascularized Tissue in Fistula Prevention
Given the preponderance of having primary chemoradiation for locally advanced laryngeal cancer based on the results of the Veteran's Affairs RTOG trials, postradiation salvage laryngeal surgery for persistent or recurrent disease remains a common indications for laryngectomy or laryngopharyngectomy and one of the most frequent etiologies of postoperative pharyngocutaneous fistulas, with rates commonly ranging from 20 to 35% in patients having salvage surgery. 10 While primary closure of laryngectomy or laryngopharyngectomy defects remains an option and has, rarely, been shown to have favorable outcomes, the use of vascularized tissue to close, or reinforce, surgical defects is widely considered the ideal method to prevent postoperative pharyngocutaneous fistula. 11 In a recent systematic review of 591 patients having salvage total laryngectomy, Paleri et al found that the use of either free or pedicled flaps decreased the incidence of postoperative pharyngocutaneous fistula by nearly 10% (31.2% vs. 22.2%) with a relative risk of 0.63 and a number needed to treat of only 11 patients. 10 Strikingly, in another review of 742 patients having salvage laryngectomy and laryngopharyngectomy, the use of a pectoralis major (PM) myocutaneous pedicled flap was associated with a 22% reduction in postoperative pharyngocutaneous fistula rates. 12 In one large multicenter series, the use of vascular tissue carried a 12% reduction in fistula formation in the reconstruction of laryngectomy-alone defects and a 31% decrease in fistula rate for laryngectomy and partial pharyngectomy. 13
The reconstructive options include either pedicled or free flaps and, generally speaking, are used to either supplement the circumferential tissue used to reconstruct the pharyngeal defect or onlayed over a primary closure of the pharyngeal defect as a manner of reinforcement. While most authors agree that the use of vascularized tissue is superior to primary closure alone in preventing postoperative fistulas, whether an ideal flap type or donor site exists, remains debated. Common flaps include the PM myocutaneous or myofascial pedicle flap, the radial forearm free flap, the anterolateral thigh free flap, and the jejunal free flap. Less frequently, the deltopectoral and supraclavicular island flaps have also been described for closure of laryngectomy and laryngopharyngectomy defects. While pedicled flaps avoid the technical difficulty and prolonged operative times associated with microvascular anastomosis, there exists little literature directly comparing their efficacy relative to free tissue transfer in preventing head and neck fistula formation. Perhaps the more pertinent and pressing question is regarding the preventative benefit of muscular tissue—in addition to a cutaneous layer—in the reconstruction of laryngopharyngeal defects. Patel et al found in a retrospective review of 359 patients that a pectoralis myocutaneous flap was superior in preventing fistula over cutaneous free flaps (15% vs. 25%). 2 Similarly, Tan et al and López et al found the anterolateral thigh free flap to have lower fistula rates than the radial forearm free flap following reconstruction of total laryngopharyngectomy defects (33% vs. 50%; 3% vs. 16%). 14 15 However, in a large, multicenter retrospective review covering 33 institutions, the Microvascular Committee of the American Academy of Otolaryngology—Head & Neck Surgery found that there was no consistent benefit for either cutaneous only or myocutaneous vascular tissue reconstruction of either laryngectomy, laryngectomy and partial pharyngectomy, or total laryngopharyngectomy defects. 13
Similarly, given the predominantly retrospective nature of the literature, few studies have directly examined the benefits of the different methods of closure. In a series of 37 patients having salvage laryngectomy, the use of cutaneous free flaps to patch the pharyngeal defect was associated with a 32% reduction in the rate of postoperative fistula formation (18% vs. 50%). 16 Correspondingly, Powell et al reported a 0% fistula rate following patch-type closure with either free or pedicled flaps, compared with a 26% rate in the cohort undergoing primary closure. 17 The onlay technique of reinforcing primary pharyngeal closure with muscular tissue utilizing PM pedicled flaps has been found to reduce fistula rates by 26% in some series. 18 In patients developing postoperative fistulas, onlay closure has also been associated with faster healing and lower rates of surgical repair of fistulas. 2 19 20 21
It should be noted that significant potential for bias can be found in the existing literature, which is comprised mainly of retrospective cohort and case series—often by single surgeons. These limited samples predispose the available data to be distorted by individual surgeon expertise and experience. Additionally, the lack of randomization raises the possibility of confounding based on individual patient and diseases differences, as it is likely that reconstructive options would be escalated or altered based on surgeon perception of risk for postoperative complications and individual patient morbidity.
Conservative Management
Despite advances in surgical technique and a variety of described vascular flaps for reconstruction of postoperative fistulas, conservative management remains the mainstay of the treatment paradigm for head and neck fistulas. Hyman et al reported that 81% of fistulas developing within the first 30 days of surgery resolved with conservative wound care alone, while a surprising 50% of fistulae presenting beyond postoperative day 30 also responded to conservative therapy. 22 In a recent systematic review, primary conservative therapy was found to have a pooled effectiveness of 56 to 90% in patients with head and neck fistulae. 23 As it has long been known that many fistulas will close with appropriate wound care, and foundations of conservative fistula management are well established, it is advisable to avoid intraoral feedings with concurrent enteral or parenteral nutrition, antibacterial therapy, routine debridement, and pressure dressings or packing. 24 Minimizing contamination of the surrounding tissues is paramount to optimal healing of fistula tracts and, as such, patients should remain strictly nothing per os for the duration of therapy. While no consensus exists regarding the endpoint after which intraoral feeds may be resumed, many surgeons recommend radiographic proof of fistula closure with a negative barium swallow prior to initiation of feeds. Optimization of nutritional status remains critical to adequate wound healing and, as such, enteral feedings via nasogastric tube—or if contraindications to enteral feedings exist, parenteral nutrition—are an essential component of fistula management and monitoring of albumin and prealbumin levels is routinely recommended. 23 In an effort to reduce drainage of secretions into the fistula tract, some authors also recommend placement of a salivary bypass tube. 25 Similar to nutritional status, the patient's volume status should be closely monitored in the healing period, with maintenance of euvolemic through intravenous fluids or transfusions as needed as fistula have been shown to cause fluid losses similar to those of a large burn. 24
Routine wound care forms the other pillar of conservative fistulae care. It is widely accepted that the fistula and surrounding tissues should be cleaned of excessive secretions frequently, that the exposed tissues be cleaned thoroughly, that the wound bed should be debrided regularly, and that adequate pressure dressing or packing be applied to the tract. Many topical therapies have been advocated by various authors, from sterile saline, to hydrogen peroxide, Dakin's solution, povidone-iodine, antibiotic impregnated saline, to zinc peroxide; however, little in the way of direct comparison of the effectiveness between various wound cleansing methods exists and it is likely that the routine practice of cleansing the wound provides much of the benefit gained. Similarly, no studies have directly assessed the effect of frequency of wound debridement on fistula healing; however, many authors recommend at a minimum use of daily mechanical debridement with wet-to-dry dressings for optimal effect. 23 While the classic wet-to-dry dressing with saline-impregnated gauze remains the workhorse of many conservative regimens, many other dressings have been suggested based on potential benefits to wound healing. Both hydrocolloid dressings and hydrogel pastes have been considered as alternative dressings in the setting of head and neck fistulae based on in vitro properties of increased exudate absorbance. 6 While some series have shown promising results, with rapid fistula resolution with the addition of hydrogel pastes to local wound care regimen, the high output nature of most head and neck fistula remains problematic for even the most advanced dressing product. 6 26 Antimicrobial products, such as silver or honey-impregnated bandages, have also been suggested based on promising results in some series and low risk of complications; however, robust, reproducible results remain lacking in this area as well. 27 28
The main areas of question in the realm of conservative fistula management surround the indications for primary operative intervention and the duration of conservative therapy required before consideration of escalation of management. As has been the theme, robust head-to-head evidence is lacking, with many recommendations being based on cohort and retrospective data. Recommendations to proceed with primary surgery have been suggested for “large” fistula, those with major wound breakdown, or exposure of vessels. 24 29 Fig. 2 demonstrates laryngectomy defect of prior described patient after total laryngectomy with debridement of chondroradionecrosis and infection. Given the presence of great vessel exposure and extensive chondroradionecrosis, conservative treatment was not an option in this case. Hyman et al, while broadly supporting initial conservative management, recommend that for late (> 30 days postoperatively) fistula signs of induration or infection being managed operatively rather than conservatively. 22 Additionally, while conservative management has been shown to be less successful in patients with a previous history of radiation (44–82%) compared with nonirradiated patients (80–95%), an initial trial of conservative therapy in radiation patients is generally indicated. 23 As to the length of primary conservative therapy, while many authors agree on an initial duration of 1 month, many reports of, successful, extended trials of conservative management lasting up to 18 weeks have also been published. 23 Notably, in their series, Hyman et al reported fistula closure to take an average of 61 days. 22 Conversely, Iteld and Yu detail markedly easier reconstructive surgeries in patients having early (less than 30 days) compared with late surgical repair of pharyngocutaneous fistulae, which forms the basis for their recommendation of only short trials of conservative therapy before proceeding with operative repair of fistulae. 30 Needless to say, the decision to continue with extended durations of conservative management versus proceed with surgery is fraught with inherent patient and surgeon-specific variability that will shape each conclusion on a case-by-case basis. Regardless, it is the opinion of these authors that conservative management of head and neck fistulae remains an ideal option in most patients.
Fig. 2.
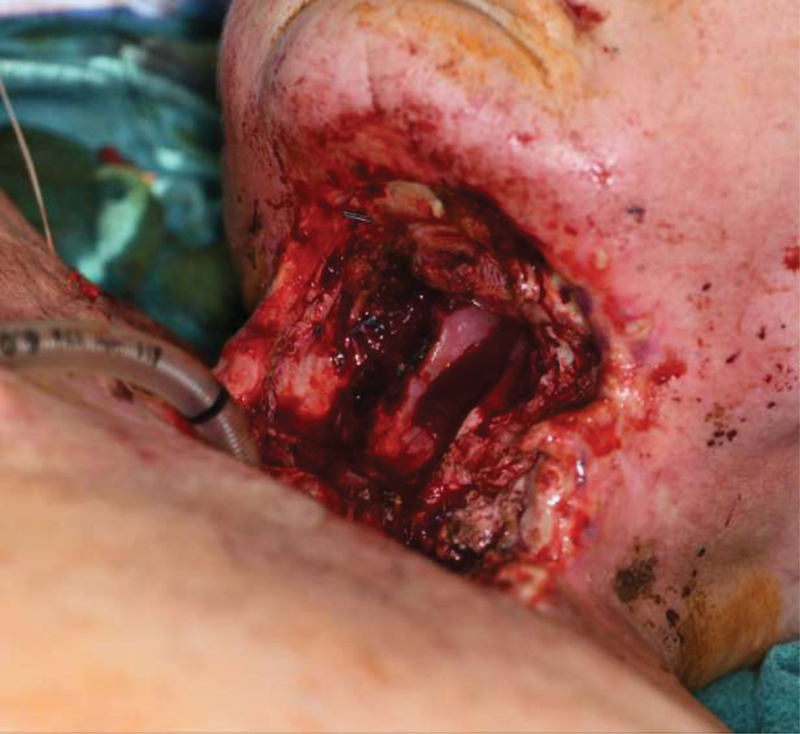
Large pharyngolaryngectomy defect following total laryngectomy with debridement of chondroradionecrosis and infection.
Negative Pressure Wound Therapy
The use of negative pressure wound therapy (NPWT), or “wound vacs,” is sometimes used in head and neck fistula management. There are multiple theoretical benefits to NPWT, including increased blood flow to the wound, constant removal of purulence or fluid from the wound bed, and ultimately the accelerated healing of the wound. Although literature regarding the effectiveness of NPWT compared with other methods of head and neck fistula closure is somewhat limited, there are reports of successful fistula closure using this method.
In a study by Inatomi et al, 28 out of 32 patients who failed more conservative measures were successfully treated with NPWT alone, without the need for surgical intervention. The mean time to fistula closure was 30.4 days, and did not differ with previously irradiated patients. 31 A systematic review by Lin et al also revealed success with NPWT. Although the exact method of NPWT varied, reported fistula closure rate ranged from 78 to 100% between studies. Several studies included in the review did include additional procedures after cessation of NPWT, including surgical treatment and conventional wound dressing. 32 Nevertheless, the use of negative pressure therapy was found to be a low risk tool for the management of head and neck fistulas in a large number of patients.
Surgical Management
When more conservative management fails, various surgical options exist to aid in the closure of head and neck fistulas. Surgical debridement with vascularized tissue coverage is often employed with varying rates of success. Specific tumor sites and prior modes of treatment likely play a role in the likelihood of need of surgical management.
In one study by Busoni et al, total laryngectomy patients who required regional flap coverage of fistula sites were analyzed. Patients who had previously underwent chemoradiation were found to be the most likely to undergo regional tissue transfer. Patients who underwent primary laryngectomies were more likely to require conservative treatment only; postradiation laryngectomy patients fell somewhere in between, requiring regional flaps more often than primary laryngectomy patients. 29
The PM regional flap is a popular method for surgical closure of head and neck fistulas. Although often successful, a high rate of fistula recurrence has been seen after PM flap in previously irradiated patients. 33 Some surgeons prefer vascularized free tissue transfer for tissue coverage once a patient has fistulized, although data regarding increased efficacy over regional tissue transfer including the PM flap is lacking. Fig. 3 demonstrates the prior discussed patient with pharynx reconstructed with a pectoralis myocutaneous flap. Fig. 4 demonstrates the anterior neck defect reconstructed with a large deltopectoral flap as patient had extensively vessel depleted necks bilaterally with ligation of the common carotid artery on the left for carotid blowout.
Fig. 3.
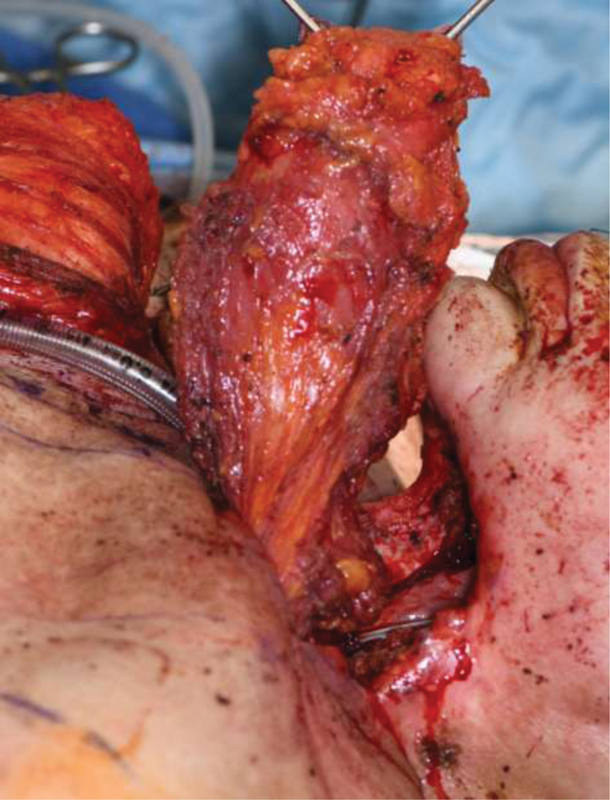
Given vessel depleted neck in prior described patient, a left pectoralis myocutaneous flap was utilized for pharyngeal reconstruction.
Fig. 4.
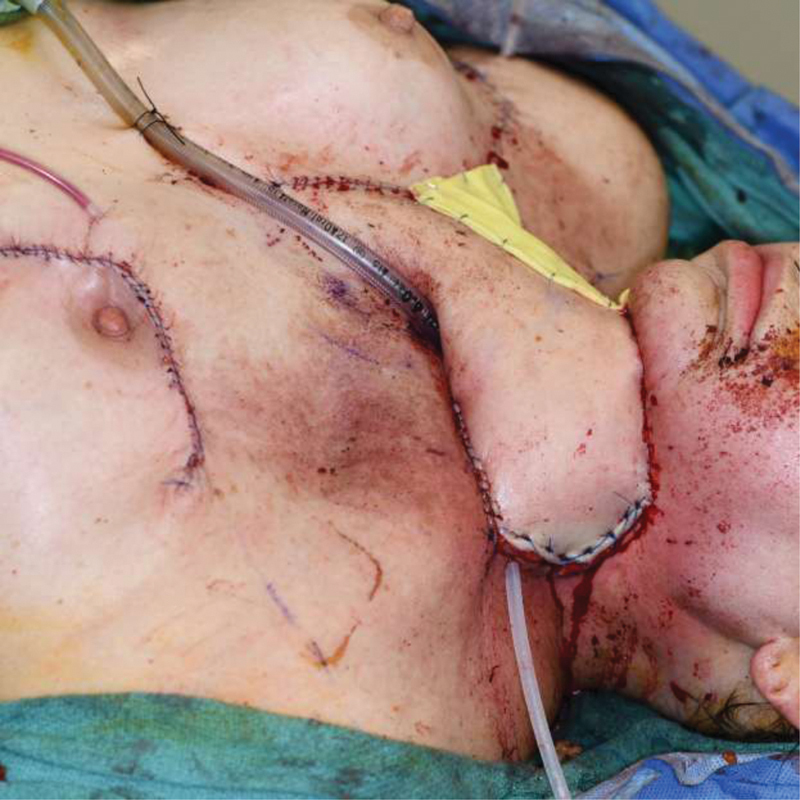
A large deltopectoral flap was utilized for external skin covering of anterior neck defect over the pectoralis flap reconstruction.
A more novel method for tissue coverage is described by Salgado et al, in which a “tissue plug” concept is employed. In this method, the dermal component from a regional flap such as the PM flap or deltopectoral flap is guided through the fistula. The flap is secured beyond the friable tissue defect with an external bolster, and the dermal component is left to “plug” the fistula tract. 34
Conclusion
Head and neck cancer treatment is commonly plagued with fistula formation, resulting in longer hospital stays, long-term antibiotic treatment, surgical repair, and even free flap failure. A patient's previous medical history such as diabetes, malnutrition, anemia, and hypothyroidism might all play a role in increasing the likelihood of developing a fistula at some point in the patient's treatment. Early recognition and treatment is of great importance to the head and neck or reconstructive surgeon, and knowledge regarding the possible treatment paths is crucial.
As many fistulas may heal spontaneously, initial conservative treatment with local wound care and antibiotics is prudent. NPWT may even be considered. Vascularized tissue coverage may be beneficial both in prevention and treatment of head and neck fistulas both before or after they arise, although ideal methods and timing are up for debate.
Footnotes
Conflict of Interest None declared.
References
- 1.Virtaniemi J A, Kumpulainen E J, Hirvikoski P P, Johansson R T, Kosma V M. The incidence and etiology of postlaryngectomy pharyngocutaneous fistulae. Head Neck. 2001;23(01):29–33. [PubMed] [Google Scholar]
- 2.Patel U A, Moore B A, Wax M. Impact of pharyngeal closure technique on fistula after salvage laryngectomy. JAMA Otolaryngol Head Neck Surg. 2013;139(11):1156–1162. doi: 10.1001/jamaoto.2013.2761. [DOI] [PubMed] [Google Scholar]
- 3.Dedivitis R A, Aires F T, Cernea C R, Brandão L G. Pharyngocutaneous fistula after total laryngectomy: systematic review of risk factors. Head Neck. 2015;37(11):1691–1697. doi: 10.1002/hed.23804. [DOI] [PubMed] [Google Scholar]
- 4.Klozar J, Cada Z, Koslabova E. Complications of total laryngectomy in the era of chemoradiation. Eur Arch Otorhinolaryngol. 2012;269(01):289–293. doi: 10.1007/s00405-011-1598-7. [DOI] [PubMed] [Google Scholar]
- 5.Mattioli F, Bettini M, Molteni G. Analysis of risk factors for pharyngocutaneous fistula after total laryngectomy with particular focus on nutritional status. Acta Otorhinolaryngol Ital. 2015;35(04):243–248. [PMC free article] [PubMed] [Google Scholar]
- 6.Khanh N T, Iyer G. Management of post-operative fistula in head and neck surgery: sweeping it under the carpet? World J Otorhinolaryngol. 2015;5(04):93–104. [Google Scholar]
- 7.Basheeth N, O'Leary G, Sheahan P. Pharyngocutaneous fistula after salvage laryngectomy: impact of interval between radiotherapy and surgery, and performance of bilateral neck dissection. Head Neck. 2014;36(04):580–584. doi: 10.1002/hed.23337. [DOI] [PubMed] [Google Scholar]
- 8.Rosko A J, Birkeland A C, Bellile E. Hypothyroidism and wound healing after salvage laryngectomy. Ann Surg Oncol. 2018;25(05):1288–1295. doi: 10.1245/s10434-017-6278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girkar F, Thiagarajan S, Malik A. Factors predisposing to the development of orocutaneous fistula following surgery for oral cancer: experience from a tertiary cancer center. Head Neck. 2019;41(12):4121–4127. doi: 10.1002/hed.25951. [DOI] [PubMed] [Google Scholar]
- 10.Paleri V, Drinnan M, van den Brekel M WM. Vascularized tissue to reduce fistula following salvage total laryngectomy: a systematic review. Laryngoscope. 2014;124(08):1848–1853. doi: 10.1002/lary.24619. [DOI] [PubMed] [Google Scholar]
- 11.Benson E M, Hirata R M, Thompson C B. Pharyngocutaneous fistula after total laryngectomy: a single-institution experience, 2001-2012. Am J Otolaryngol. 2015;36(01):24–31. doi: 10.1016/j.amjoto.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guimarães A V, Aires F T, Dedivitis R A. Efficacy of pectoralis major muscle flap for pharyngocutaneous fistula prevention in salvage total laryngectomy: a systematic review. Head Neck. 2016;38 01:E2317–E2321. doi: 10.1002/hed.24248. [DOI] [PubMed] [Google Scholar]
- 13.Microvascular Committee of the American Academy of Otolaryngology-Head & Neck Surgery* . Salvage laryngectomy and laryngopharyngectomy: Multicenter review of outcomes associated with a reconstructive approach. Head Neck. 2019;41(01):16–29. doi: 10.1002/hed.25192. [DOI] [PubMed] [Google Scholar]
- 14.Tan N C, Lin P Y, Kuo P J. An objective comparison regarding rate of fistula and stricture among anterolateral thigh, radial forearm, and jejunal free tissue transfers in circumferential pharyngo-esophageal reconstruction. Microsurgery. 2015;35(05):345–349. doi: 10.1002/micr.22359. [DOI] [PubMed] [Google Scholar]
- 15.López F, Obeso S, Camporro D, Fueyo A, Suárez C, Llorente J L. Outcomes following pharyngolaryngectomy with fasciocutaneous free flap reconstruction and salivary bypass tube. Laryngoscope. 2013;123(03):591–596. doi: 10.1002/lary.23695. [DOI] [PubMed] [Google Scholar]
- 16.Withrow K P, Rosenthal E L, Gourin C G. Free tissue transfer to manage salvage laryngectomy defects after organ preservation failure. Laryngoscope. 2007;117(05):781–784. doi: 10.1097/MLG.0b013e3180332e39. [DOI] [PubMed] [Google Scholar]
- 17.Powell J, Ullal U, Ahmed O, Ragbir M, Paleri V. Tissue transfer during salvage laryngectomy following chemoradiation to prevent pharyngocutaneous fistula. J Laryngol Otol. 2014;128(04):365–367. doi: 10.1017/S0022215114000504. [DOI] [PubMed] [Google Scholar]
- 18.Righini C A, Bettega G, Lequeux T, Chaffanjeon P, Lebeau J, Reyt E. Use of tubed gastro-omental free flap for hypopharynx and cervical esophagus reconstruction after total laryngo-pharyngectomy. Eur Arch Otorhinolaryngol. 2005;262(05):362–367. doi: 10.1007/s00405-004-0828-7. [DOI] [PubMed] [Google Scholar]
- 19.Gil Z, Gupta A, Kummer B. The role of pectoralis major muscle flap in salvage total laryngectomy. Arch Otolaryngol Head Neck Surg. 2009;135(10):1019–1023. doi: 10.1001/archoto.2009.126. [DOI] [PubMed] [Google Scholar]
- 20.Fung K, Teknos T N, Vandenberg C D. Prevention of wound complications following salvage laryngectomy using free vascularized tissue. Head Neck. 2007;29(05):425–430. doi: 10.1002/hed.20492. [DOI] [PubMed] [Google Scholar]
- 21.Hanasono M M. Use of reconstructive flaps following total laryngectomy. JAMA Otolaryngol Head Neck Surg. 2013;139(11):1163. doi: 10.1001/jamaoto.2013.2768. [DOI] [PubMed] [Google Scholar]
- 22.Hyman J, Disa J J, Cordiero P G, Mehrara B J.Management of salivary fistulas after microvascular head and neck reconstruction Ann Plast Surg 20065703270–273., discussion 274 [DOI] [PubMed] [Google Scholar]
- 23.Molteni G, Sacchetto A, Sacchetto L, Marchioni D. Optimal management of post-laryngectomy pharyngo-cutaneous fistula. Open Access Surgery. 2020;13:11–25. [Google Scholar]
- 24.Myers E N. The management of pharyngocutaneous fistula. Arch Otolaryngol. 1972;95(01):10–17. doi: 10.1001/archotol.1972.00770080058003. [DOI] [PubMed] [Google Scholar]
- 25.Kwon D, Genden E M, de Bree R. Overcoming wound complications in head and neck salvage surgery. Auris Nasus Larynx. 2018;45(06):1135–1142. doi: 10.1016/j.anl.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Diallo B K, Lacher-Fougere S, Baltazart B, Traissac L, Houliat T.Results of alginate and hypertonic solution in wound healing of head and neck cancers [in French] Rev Laryngol Otol Rhinol (Bord) 2008129(4-5):289–292. [PubMed] [Google Scholar]
- 27.Ganacias-Acuna E F. Active Leptospermum honey and negative pressure wound therapy for nonhealing postsurgical wounds. Ostomy Wound Manage. 2010;56(03):10–12. [PubMed] [Google Scholar]
- 28.Cooper R. Impact of honey as a topical treatment for wounds remains unclear. Evid Based Med. 2014;19(01):11. doi: 10.1136/eb-2013-101331. [DOI] [PubMed] [Google Scholar]
- 29.Busoni M, Deganello A, Gallo O. Pharyngocutaneous fistula following total laryngectomy: analysis of risk factors, prognosis and treatment modalities. Acta Otorhinolaryngol Ital. 2015;35(06):400–405. doi: 10.14639/0392-100X-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iteld L, Yu P. Pharyngocutaneous fistula repair after radiotherapy and salvage total laryngectomy. J Reconstr Microsurg. 2007;23(06):339–345. doi: 10.1055/s-2007-992343. [DOI] [PubMed] [Google Scholar]
- 31.Inatomi Y, Kadota H, Yoshida S. Utility of negative-pressure wound therapy for orocutaneous and pharyngocutaneous fistula following head and neck surgery. Head Neck. 2020;42(01):103–110. doi: 10.1002/hed.25989. [DOI] [PubMed] [Google Scholar]
- 32.Lin F Y, Huang P Y, Cheng H T. Systematic review of negative pressure wound therapy for head and neck wounds with fistulas: outcomes and complications. Int Wound J. 2020;17(02):251–258. doi: 10.1111/iwj.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLean J N, Nicholas C, Duggal P. Surgical management of pharyngocutaneous fistula after total laryngectomy. Ann Plast Surg. 2012;68(05):442–445. doi: 10.1097/SAP.0b013e318225832a. [DOI] [PubMed] [Google Scholar]
- 34.Salgado C J, Mardini S, Chen H C, Chen S. Critical oropharyngocutaneous fistulas after microsurgical head and neck reconstruction: indications for management using the “tissue-plug” technique. Plast Reconstr Surg. 2003;112(04):957–963. doi: 10.1097/01.PRS.0000076219.62225.07. [DOI] [PubMed] [Google Scholar]


