Figure 7.
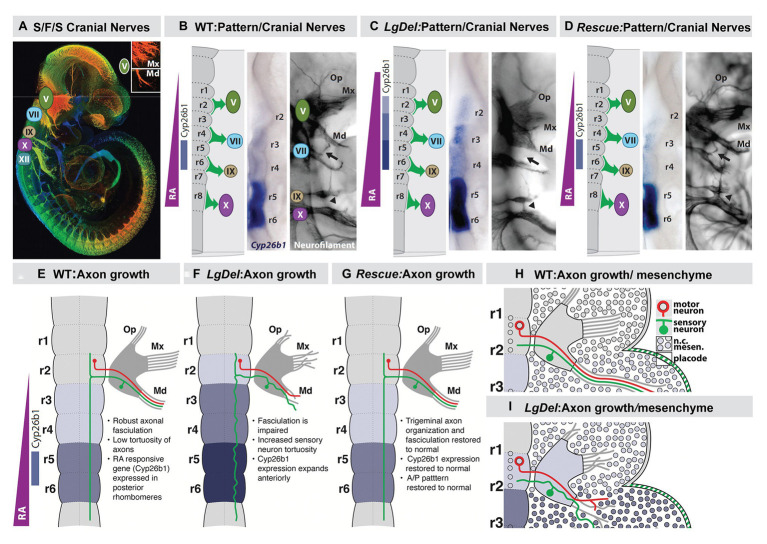
Early disruption of hindbrain patterning alters anterior cranial nerve differentiation, prefiguring anomalous oropharyngeal sensory/motor function that likely contributes to suckling, feeding, and swallowing (S/F/S) difficulties in early post-natal LgDel mouse pups who carry a heterozygous deletion of the 28 murine orthologues of the genes deleted in 22q11DS. (A) The five cranial nerves that contribute to sensory/motor control of S/F/S have begun to differentiate by E10.5 in the mouse. In this preparation, they have been immunolabeled in the whole by the early marker for neuron and axons, βIII-tubulin, and visualized in a high-resolution confocal image in which embryo volume/depth is color coded, with warm colors representing structures close to the viewer and cooler colors representing those deeper in the embryo. The inset shows the multiple small axon fascicles that characterize the maxillary branch (Mx) of the trigeminal nerve (V) and the single fascicle of axons that forms as the mandibular branch in typically developing WT embryos. (B) The A-P array of S/F/S contributing cranial nerves is prefigured in E9.5 embryos by a gradient of RA-signaling that distinguishes posterior (r5,6) from anterior (r2,3) rhombomeres in the developing hindbrain. This posterior RA-dependent patterning, as well as opposing anterior signaling via Fgfs and Wnts, specifies the precursors of the cranial sensory neurons and hindbrain motor neurons that then differentiate as the cranial nerves within 24 h. (C) In LgDel E9.5 embryos, the gradient of RA signaling is enhanced in and shifted beyond posterior rhombomeres; it now elicits RA-regulated gene expression in anterior rhombomeres. Within a day, anterior cranial nerves, V (trigeminal) and VII (facial) are dysmorphic. The multiple axon fascicles normally seen in the Mx of V are diminished, the mandibular branch is similarly hypotrophic, and the facial nerve (VII) lacks its nascent anterior branch (arrow). In addition, the posterior cranial nerves IX (glossopharyngeal) and X (vagus) have either small axonal anastomoses (arrowhead) or in extreme cases are fused. (D) When RA signaling levels are diminished genetically by heterozygous deletion of the RA synthetic gene Raldh2 in LgDel embryos (“Rescue”), the pattern of RA-dependent gene expression in the anterior rhombomeres returns to that seen in the WT. In parallel, initial differentiation of the nascent trigeminal and facial nerve is restored to the WT state. The ophthalamic (Op), Mx, and mandibular branches of the trigeminal nerve (V) extend toward their targets as in the WT with similar degrees of fasciculation. The facial nerve branches appropriately (arrow). The fusion of the posterior cranial nerves IX and X persists, most likely because this reflects the disrupted differentiation of cardiovascular targets due to Tbx1 heterozygous deletion, independent of hindbrain RA-dependent A-P patterning. (E–H) Schematics of the relationship between RA-dependent hindbrain patterning and the growth and trajectory of individual trigeminal motor and sensory axons in the WT embryo. Individual trigeminal motor axons, as well as primarily placodal derived trigeminal sensory axons, respond differently as they interact with neural crest derived mesenchymal substrates in the periphery whose A-P identity has been presumably altered by enhanced RA signaling in the anterior rhombomeres.