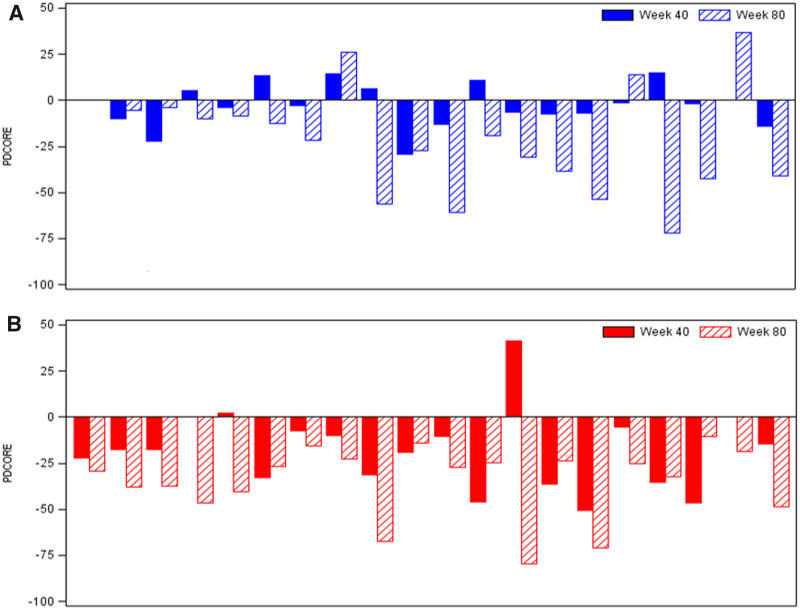
Figure 3.
Intra-patient change in the final PDCORE. The final PDCORE was calculated as 1x + 10.8/5.2y − 10.8/1.67z = 1x + 2.08y − 6.47z (x: change from baseline in OFF state UPDRS motor score; y: change from baseline in OFF state UPDRS ADL score; z: change in total good-quality ON time per day). Negative PDCORE values are indicative of clinical improvement. (A) Placebo/GDNF sequence. Patients received placebo between baseline and Week 40 (randomized, double-blind), followed by GDNF between Week 40 and Week 80 (open-label) (Whone et al., 2019a, b). Two patients had missing values for good-quality ON time at Week 40 and one at Week 80 and were excluded from the respective analyses. The distribution of the PDCORE values across placebo patients at Week 40 appears to be heterogeneous, with limited response in approximately half of the patients. In contrast, at Week 80 (placebo/GDNF), a more homogeneous pattern of negative PDCORE values emerges. (B) GDNF/GDNF sequence. Patients received GDNF both between baseline and Week 40 (randomized, double-blind) and between Week 40 and Week 80 (open-label) (Whone et al., 2019a, b). One patient was involved in a car accident during the study and was excluded from the analysis due to a conus injury preventing several parts of the UPDRS at different time-points from being completed. Two other patients had missing values for good-quality ON time at Week 40 and were excluded from the analysis. The distribution of the PDCORE values across GDNF patients at Week 40 appears to be similar to the pattern observed in patients of the placebo/GDNF sequence at Week 80, with further improvements incurred by Week 80. Of note, all patients in the GDNF/GDNF sequence had a negative PDCORE value (indicating clinical improvement) at Week 80.