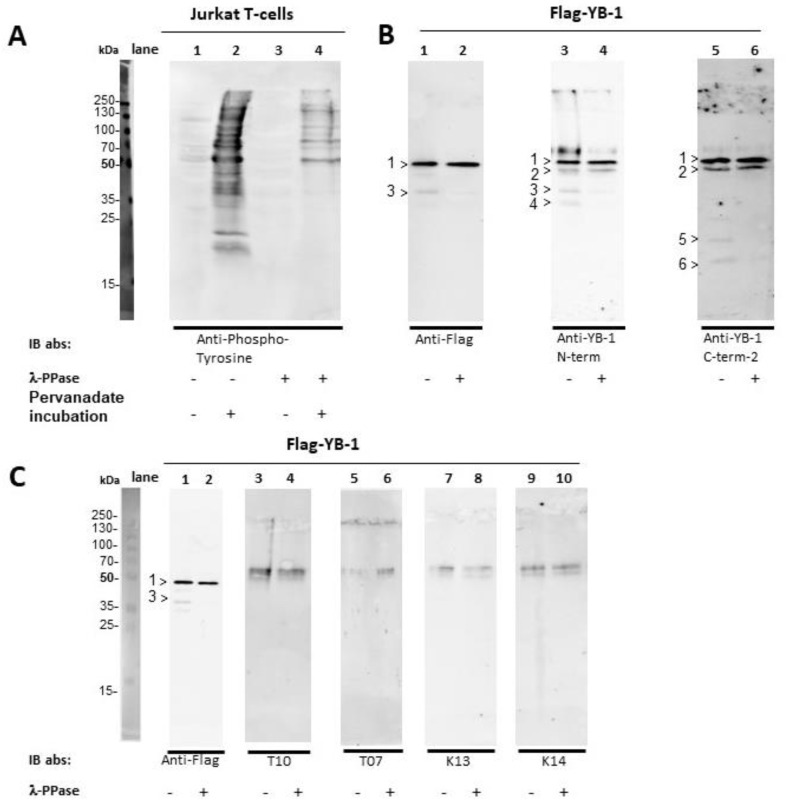
Figure 4.
Dephosphorylation of Jurkat E6 T-cells and eukaryotic Flag-YB-1 Jurkat E6 T-cell extracts were prepared in the presence and absence of irreversible phosphatase inhibitor pervanadate. In a second step, the cell lysates, as well as the Flag-YB-1 protein, were incubated for 24 h at 30 °C with λ-phosphatase for dephosphorylation. Flag-YB-1 without the addition of λ-phosphatase was also incubated at 30 °C for 24 h. (A) About half a million cells were used for SDS-PAGE. Anti-phosphotyrosine antibody [4G10] was applied to Jurkat T-cells to demonstrate λ-phosphatase activity (lane 1–4). (B) To determine potential cleavage of Flag-YB-1 protein during incubation 1.5 µg of Flag-YB-1 was used for SDS-PAGE. Flag specific, as well as N-terminal and C-terminal targeting antibodies, were applied. Untreated Flag-YB-1 shows 2 N-terminal fragments at about 40 kDa and 35 kDa (lane 3, >3 and >4) with 2 corresponding C-terminal fragments (lane 5, >5 and >6). Fragment >2 (50 kDa) probably reflects untagged eukaryotic YB-1 as it is not detected by Flag specific antibodies. Dephosphorylated Flag-YB-1 presents a retarded fragmentation (compare lanes 1, 3, 5 with 2, 4, and 6). (C) 1.5 µg of untreated and dephosphorylated Flag-YB-1 protein were probed with a serum of tumor patients (lane 3–6) and healthy controls (lane 7–10). Flag specific antibody served as control (lane 1–2). All serum samples show unspecific reactions against lanes containing only sample buffer (compare original blots). None of the serum samples presents autoantibodies against eukaryotic Flag-YB-1.