Abstract
To differentiate white wines from Croatian indigenous varieties, volatile aroma compounds were isolated by headspace solid-phase microextraction (HS-SPME) and analyzed by comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry (GC×GC-TOF-MS) and conventional one-dimensional GC-MS. The data obtained were subjected to uni- and multivariate statistical analysis. The extra separation ability of the GC×GC second dimension provided additional in-depth volatile profile information, with more than 1000 compounds detected, while 350 were identified or tentatively identified in total by both techniques, which allowed highly efficient differentiation. A hundred and sixty one compounds in total were significantly different across monovarietal wines. Monoterpenic compounds, especially α-terpineol, followed by limonene and linalool, emerged as the most powerful differentiators, although particular compounds from other chemical classes were also shown to have notable discriminating ability. In general, Škrlet wine was the most abundant in monoterpenes, Malvazija istarska was dominant in terms of fermentation esters concentration, Pošip contained the highest levels of particular C13-norisoprenoids, benzenoids, acetates, and sulfur containing compounds, Kraljevina was characterized by the highest concentration of a tentatively identified terpene γ-dehydro-ar-himachalene, while Maraština wine did not have specific unambiguous markers. The presented approach could be practically applied to improve defining, understanding, managing, and marketing varietal typicity of monovarietal wines.
Keywords: two-dimensional gas chromatography, one-dimensional, wine, volatile aroma compounds, multivariate analysis, cultivar, Croatia
1. Introduction
Aroma is among the most important attributes that drive the perception of wine sensory quality and varietal typicity by consumers. It results from the occurrence of many diverse odoriferous volatile compounds of different origin. Primary or varietal aroma compounds originate from grapes, secondary or fermentation aroma compounds are produced in fermentation, while tertiary aromas are formed during maturation [1,2,3]. The three groups mentioned are not so clearly divided: most of the precursors of volatile aroma compounds originate from grapes and are in one way or another affected by fermentation and/or aging [4]. The final wine aroma profile is a result of complex interactive effects between many sources of variability, such as variety [5], geographical position characterized by specific agroecological conditions [6,7], viticultural practices [8], harvest date [9], harvest year [10,11], grape processing, and fermentation parameters [12,13], etc.
Varietal characterization (description) and differentiation (contradistinction from other varieties) is an ever-important field of wine research. Many studies have aimed to identify volatile compounds characteristic for various grape varieties, since they are crucial for the typical varietal attributes of their wines. The knowledge on the volatile aroma compound composition of monovarietal wines is important since it may enable producers to better cope with the phenomena encountered in production and to manage vinification with greater efficiency, all in order to produce high quality wines of accentuated varietal typicity. It may enable detailed and precise description of the aroma of monovarietal wines, which could be used in their marketing, especially towards informed consumers interested in wines of high quality with marked diversity and identity. In addition to often being linked to a given geographical provenance with a corresponding protected designation of origin (PDO), particular monovarietal wines are especially appreciated and demanded because of their typical sensory properties. Such wines often fall within a higher price range and are a target of counterfeiting by mislabeling their varietal origin. Therefore, control in terms of varietal origin authentication is needed: the general strategy used by many research groups includes the (semi)quantification of a large number of volatile compounds in large sets of wines and use of the generated data for the production of multivariate statistical models able to classify wines, as well as to predict and confirm their varietal origin [5].
The analysis of volatile aroma compounds in wine varietal characterization and differentiation studies is commonly performed by conventional one-dimensional gas chromatography mass spectrometry (GC-MS) [14,15,16,17,18,19]. Although the information obtained by this approach is often sufficient to obtain more or less efficient varietal differentiation, a large amount of information is lost due to frequent co-elutions, even when using long GC run times on high-efficiency capillary columns with selective stationary phases and programmed oven temperature conditions [20,21]. In the last few decades, comprehensive two-dimensional gas chromatography-mass spectrometry (2D-GC-MS or GC×GC-MS) stood out as a highly potent technique for in-depth characterization of complex samples [22], where the number of compounds of interest is large and many are present at trace levels, as in wine. This technique utilizes two GC columns of different stationary phases serially connected by a modulator, where the compounds co-eluting in the first column are in most cases separated in the second. GC×GC-MS is therefore characterized by higher efficiency and sensitivity, since the additional separation by a second stationary phase produces clearer mass spectra and much less chromatographic peaks remain unannotated. In this way, GC×GC-MS allows detection and identification of a much larger number of volatile compounds compared to conventional GC-MS [23].
Regardless of the existing great potential, only a few studies have utilized GC×GC to investigate wine volatile aroma profiles, while studies which used GC×GC for varietal characterization and differentiation were extremely rare. Several authors reported more or less detailed GC×GC volatile aroma profiles of particular monovarietal wines, such as Cabernet Sauvignon [24], Sauvignon Blanc [25], Shiraz [9,26] or Syrah [12], Pinotage [21], Chardonnay [27], and Verdicchio [28], but none of them directly compared them to or differentiated them from other monovarietal wines of similar typology. In this way, despite detailed profiles determined in some cases, it still remained unknown which compounds and in which amounts are typical for a given variety and whether they could differentiate it from other monovarietal wines. The only two studies which utilized GC×GC and succeeded in differentiating several monovarietal wines did not report actual concentrations of all the identified volatile compounds [20,29].
The aim of this study was to utilize the potential of two-dimensional gas chromatography with time-of-flight mass spectrometry (GC×GC-TOF-MS) technique, in combination with headspace solid-phase microextraction (HS-SPME) and multivariate statistical tools, as a more efficient approach to characterize and differentiate monovarietal white wines based on their volatile aroma compound composition. Profiling by GC×GC was combined with conventional GC-MS analysis of major wine volatile compounds to obtain more comprehensive aroma profiles. Special attention was devoted to terpenes, often highlighted as key varietal markers in wine. The approach was applied to characterize and differentiate Croatian wines made from indigenous grape varieties, with each variety represented by a rather heterogeneous group of wines with respect to geographical microlocation and agroecological conditions, viticultural practices, harvest date, and grape processing and wine production parameters. It was expected that GC×GC-TOF-MS would be extremely effective in providing novel in-depth information for efficient white wine varietal differentiation.
2. Materials and Methods
2.1. Wine Samples
A total of 32 wines made from Croatian indigenous white grape varieties (Vitis vinifera L.) Malvazija istarska (MI, 8 samples) Pošip (PO, 7), Maraština (MA, 7), Kraljevina (KR, 7), and Škrlet (SK, 3) were donated by producers from Croatia (EU), more specifically Istria (MI) and Dalmatia (PO and MA) as the coastal regions and continental Croatia (KR and SK). Wines from the same variety were donated by different producers. The selection was representative for Croatian wine production and comprised the majority of the most important Croatian indigenous varieties. Only young wines from harvest 2015 were collected, labelled with a protected designation of origin (PDO) and with a traditional term “Quality or Top quality” wine. Wines were of the same typology and produced by standard white winemaking technology, which included grape harvest at technological maturity, destemming, crushing and mashing of the grapes, no or short pre-fermentative skin-contact (up to 48 h), use of selected commercial yeasts, fermentation at relatively low temperatures (up to 18 °C), and other standard procedures (sulfiting, racking, fining, and stabilization, etc.). Wines were not in contact with wood. During the period from harvest and vinification in September 2015 until the collection and analyses in April and May 2016 the wines were stored in stainless steel tanks and 0.75 L glass bottles with cork stoppers in wine cellars of the producers. The wine samples were selected from a larger set as typical representatives of a given variety by the panel for wine sensory analysis of the Institute of Agriculture and Tourism in Poreč (Croatia), which consisted of highly trained and experienced tasters. Standard physico-chemical parameters of the collected wines determined by OIV methods are reported in Table S1.
2.2. Standards, Chemicals, and Consumables
Chemical standards of volatile aroma compounds were procured from AccuStandard Inc. (New Haven, CT, USA), Fluka (Buchs, Switzerland), Honeywell International Inc. (Morris Plains, NJ, USA), Merck (Darmstadt, Germany), and Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA). A stock solution of major volatile compounds commonly present in wine was prepared in methanol, while standard solutions were prepared in model wine (13 vol.% of ethanol, pH 3.3). Ammonium sulfate and sodium chloride were purchased from Kemika d.d (Zagreb, Croatia).
Divinylbenzene/carboxen/polydimethylsiloxane (DVB-CAR-PDMS, StableFlex, 50/30 μm, 1 cm) SPME fiber used for GC-MS analysis was procured from Supelco, Sigma Aldrich (Bellafonte, PA, USA) and DVB-CAR-PDMS SPME fiber (StableFlex, 50/30 μm, 2 cm) used for GC×GC-TOF-MS analysis was procured from Supelco, Sigma Aldrich (Milan, Italy).
2.3. Analysis of Volatile Aroma Compounds by Conventional One-Dimensional GC-MS
Volatile aroma compounds for GC-MS analysis were isolated by headspace solid-phase microextraction (HS-SPME) according to the modified method proposed by Bubola et al. [30]. Four milliliters of a solution obtained by diluting wine four times with deionized water were pipetted in a 10 mL glass vial. Ammonium sulfate (1 g) and 50 μL of internal standards solution (2-octanol (0.84 mg/L), 1-nonanol (0.82 mg/L), and heptanoic acid (2.57 mg/L)) were added. After 15 min preconditioning at 40 °C, microextraction using a DVB-CAR-PDMS SPME fiber took place for 40 min at 40 °C with stirring (800 rpm). Volatile compounds were desorbed after the insertion of the fiber for 10 min into a GC/MS injector heated at 248 °C, with the first 3 min in splitless mode. Volatile aroma compounds were identified and quantified using a Varian 3900 gas chromatograph (GC) connected to a Varian Saturn 2100T mass spectrometer with an ion trap analyzer (Varian Inc., Harbour City, CA, USA). The column used was a 60 m × 0.25 mm i.d. × 0.25 μm d.f. Rtx-WAX (Restek, Belafonte, PA, USA). Initial temperature of the GC oven was 40 °C, ramped up at 2 °C/min to reach 240 °C, and then kept at this temperature for additional 10 min. Helium was used as a carrier gas at a flow rate of 1.2 mL/min. Mass spectra were acquired in EI mode (70 eV), at 30–350 m/z.
Identification of volatile compounds was conducted by comparison of retention times and mass spectra of the analytes with those of pure standards, and with mass spectra from NIST05 library. Identification by comparison with mass spectra was considered satisfactory if spectra reverse match numbers (RM) higher than 800 were obtained. In the case of less clear spectra (RM < 800) identification was considered satisfactory if the ratios of the relative intensities of a quantifier ion and three characteristic ions with the highest intensity reasonably matched those in the reference spectra of a given compound. Linear retention indices were calculated with respect to the retention times of C10 to C28 n-alkanes and compared to those reported in literature for columns of equal or equivalent polarity. Calibration curves were constructed based on the analysis of standard solutions containing known concentrations of standards at six concentration levels and were used for quantification. Quantification of major volatile compounds was based on total ion current peak area, while quantification of minor compounds was based on quantifier ion peak area. The peak areas and concentrations in standard solutions and in wine samples were normalized with respect to those of the internal standards. Linearity was satisfactory with coefficient of determination higher than 0.99 for all the standards. Relative standard deviation of repeatability (RSD) was determined after repeated analysis (n = 5) of a Malvazija istarska wine sample and was satisfactory, with RSD lower than 13.05% for monoterpenes, 7.38 for β-damasenone, lower than 9.23% for alcohols, 7.34 for ethyl esters, 12.34% for acetate esters, and 11.78% for fatty acids. Method validation parameters were previously published in the study of Bubola et al. [30]. In the cases when pure chemical standards were not available, semi-quantitative analysis was carried out. The concentrations of such compounds were expressed as equivalents of compounds with similar chemical structure which were quantified using calibration curves, assuming a response factor equal to one.
2.4. Analysis of Volatile Aroma Compounds by GC×GC-TOF-MS
A volume of 2.5 mL of wine was transferred to a 20 mL headspace vial and 1.5 g of sodium chloride was added. Wine sample was spiked with 50 μL of internal standard (2-octanol, 1 mg/L). Quality control samples (QC) were prepared by mixing equal proportion of each sample and were analyzed before the samples sequence (n = 5) and after every five samples (n = 1). GC×GC-TOF-MS analysis of wines was performed using a GC Agilent 7890N (Agilent Technologies, Palo Alto, CA, USA) coupled to a LECO Pegasus IV time-of-flight mass spectrometer (TOF-MS) (Leco Corporation, St. Joseph, MI, USA) equipped with a Gerstel MPS autosampler (GERSTEL GmbH & Co. KG, Mülheim an der Ruhr, Germany), as described in previous studies with minor modifications [9,31,32]. Briefly, samples were preconditioned at 35 °C for 5 min and volatile compounds were extracted using a DVB/CAR/PDMS SPME fiber for 20 min. Volatile compounds were desorbed for 3 min at 250 °C in splitless mode. The fiber was reconditioned for 7 min at 270 °C between each extraction. Helium was used as a carrier gas at a flow rate of 1.2 mL/min. The oven was equipped with a 30 m × 0.25 mm × 0.25 μm film thickness VF-WAXms column (Agilent Technologies) in the first dimension (1D) and a 1.5 m × 0.15 mm × 0.15 μm film thickness Rxi 17Sil MS column (Restek) in the second dimension (2D). Initial oven temperature was maintained at 40 °C for 4 min, then raised at 6 °C/min to 250 °C, and then finally maintained at this temperature for additional 5 min. The second oven was maintained at 5 °C above the temperature of the first one throughout the analysis. The modulator was offset by +15 °C in relation to the secondary oven, the modulation time was 7 s with 1.4 s of hot pulse duration, as described previously [31]. Electron ionization at 70 eV was applied, the temperature of ion source was 230 °C, detector voltage was 1317 V, mass range (m/z) was 40–350, acquisition rate was 200 spectra/s, and acquisition delay was 120 s.
Baseline correction, chromatogram deconvolution and peak alignment were performed using LECO ChromaTOF software version 4.32 (Leco Corporation, St. Joseph, MI, USA). The baseline offset was set to 0.8 and signal to noise (S/N) ratio was set at 100. Peak width limits were set to 42 s and 0.1 s in the first and the second dimension, respectively. Traditional, not adaptive integration was used. The required match (similarity) to combine peaks was set to 650. Under these conditions 1025 putative compounds were detected. Volatile compounds were identified by comparing their retention times and mass spectra with those of pure standards and with mass spectra from NIST 2.0, Wiley 8, and FFNSC 2 (Chromaleont, Messina, Italy) mass spectral libraries, with a minimum library similarity match factor of 750 out of 999. For identification of compounds by comparison with pure standards, a mix of 122 compounds was injected under identical GC×GC-TOF-MS conditions. For tentative identification of compounds and/or confirmation of their identities determined as described above, linear retention indices were calculated with respect to the retention times of C10 to C30 n-alkanes and compared to those from literature for conventional one-dimensional GC obtained using columns of equal or equivalent polarity (NIST 2.0, Wiley 8, FFNSC 2, VCF, ChemSpider). Three hundred and seventeen (317) volatile aroma compounds were (tentatively) identified in total. Volatile compounds were semi-quantified and their concentrations in μg/L were calculated relative to the internal standard 2-octanol, assuming a response factor equal to one.
In preliminary tests by principal component analysis (PCA), QC samples were clustered very close and were very well separated from the wine samples, suggesting the repeatability of the method was very good. Relative standard deviation of the internal standard 2-octanol in QC samples was 10.4% which was considered satisfactory for HS-SPME/GC×GC-TOF-MS analysis.
2.5. Statistical Data Elaboration
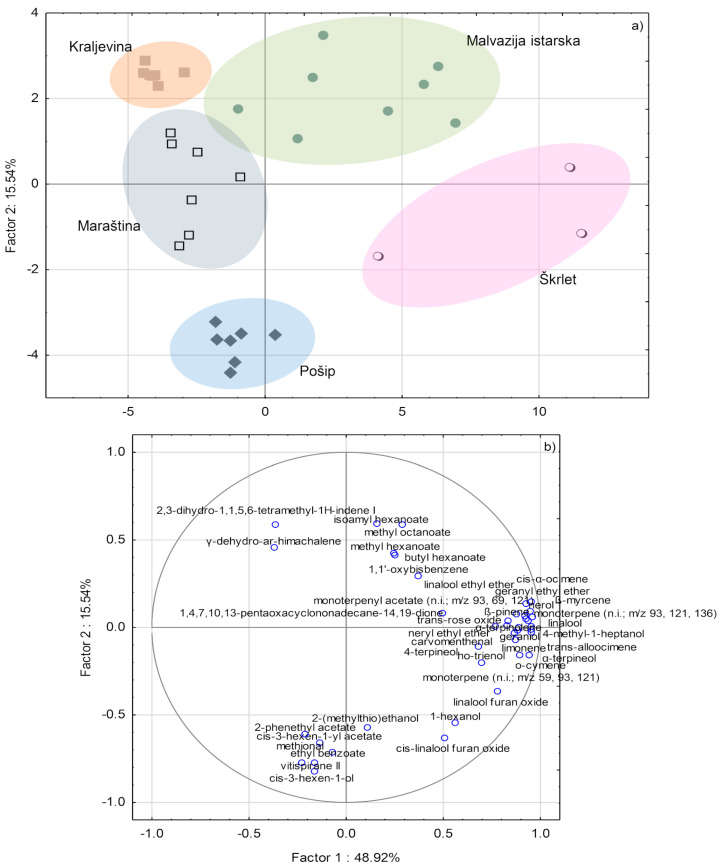
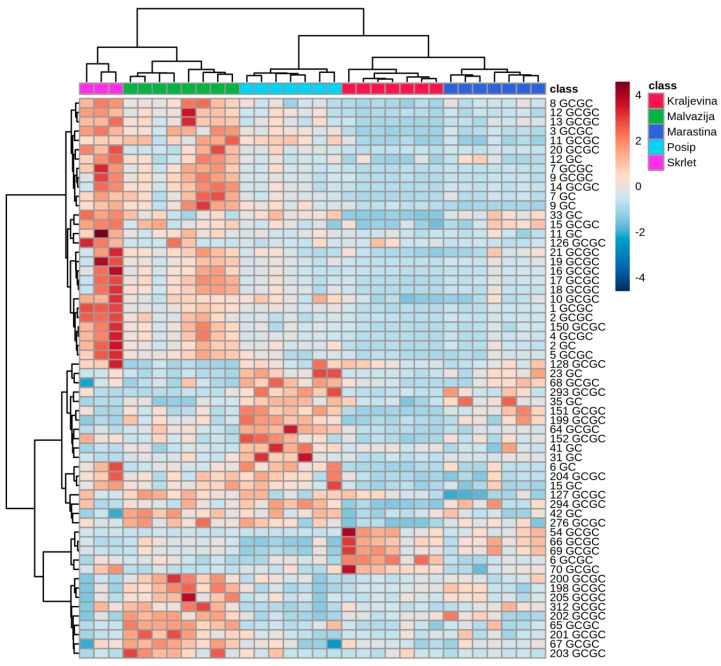
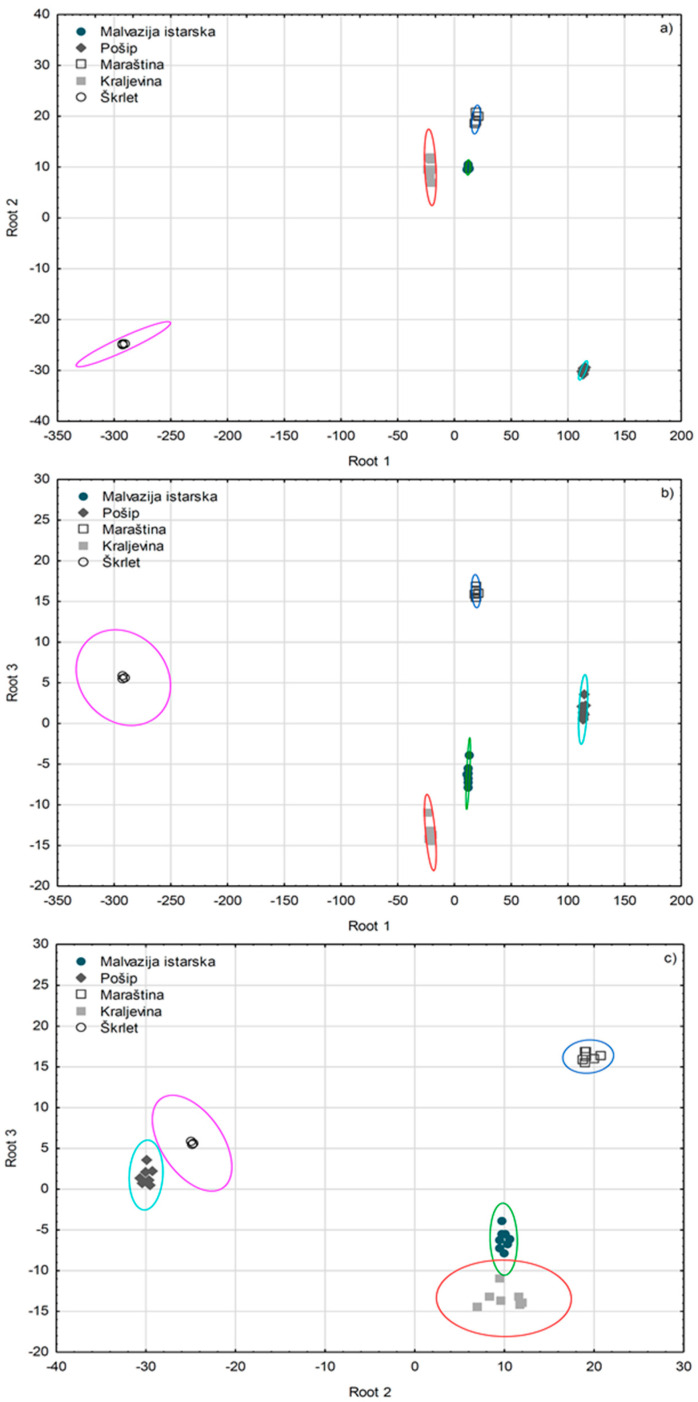
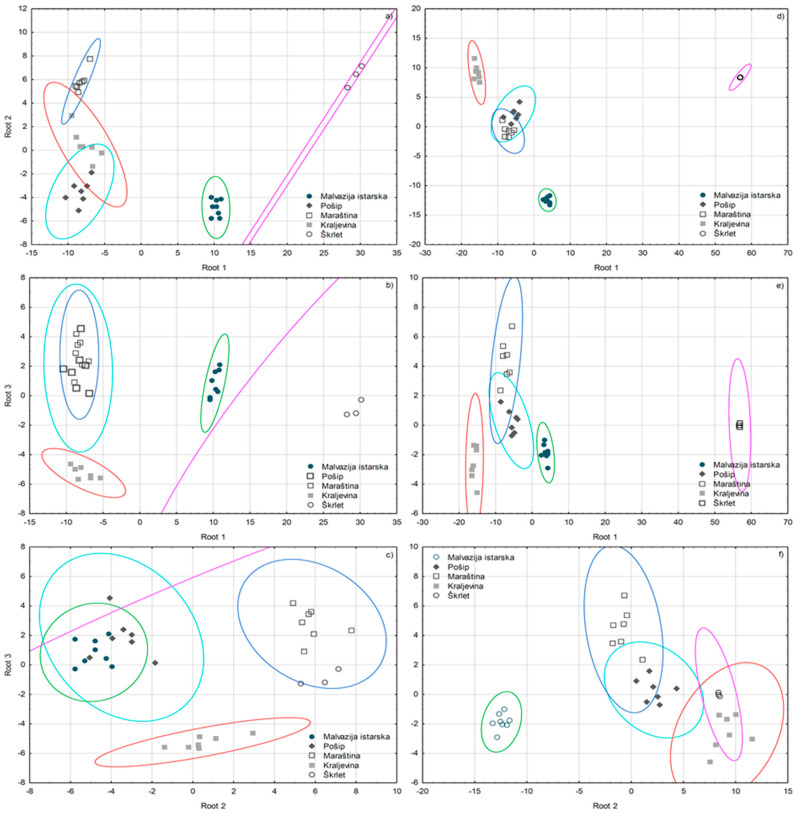
Data obtained by GC-MS and GC×GC-TOF-MS were processed by analysis of variance (one-way ANOVA). Least significant difference (LSD) post-hoc test was used to compare the mean values of concentrations at p < 0.05. Multivariate analysis of data was performed by PCA and forward stepwise linear discriminant analysis (SLDA). The original dataset which included 32 wines and 350 volatile aroma compounds (33 determined by GC-MS + 317 determined by GC×GC-TOF-MS analysis; in the case of compounds determined by both techniques GC×GC-TOF-MS data were used), was reduced based on Fisher ratios (F-ratios). Multivariate techniques were applied on the variables (mean-centered concentrations of volatile compounds) with the highest F-ratios. PCA was performed with 40 variables with the highest F-ratio, while SLDA and hierarchical clustering were performed with 60 variables with the highest F-ratio, in both cases with GC-MS and GC×GC-TOF-MS data combined. Two additional SLDA models were built with the concentrations of terpenes which were significantly different between wines, using GC-MS and GC×GC-TOF-MS data separately. In SLDA, variables were selected based on Wilk’s lambda, with F to enter = 1 and F to remove = 0.5. Cross-validation was applied to check the prediction capacity of the developed SLDA models. ANOVA, PCA, and SLDA were performed by Statistica v. 13.2 software (StatSoft Inc., Tulsa, OK, USA). Hierarchical clustering was conducted and a heatmap was generated by Ward algorithm and Euclidean distance analysis using MetaboAnalyst v. 4.0 (http://www.metaboanalyst.ca), created at the University of Alberta, Canada [33].
3. Results and Discussion
3.1. GC-MS
Major volatile aroma compounds are highly abundant in wines and for this reason GC-MS was considered appropriate for their analysis. It was considered that their quantitation by GC-MS was not significantly affected by co-eluting compounds. As well, the analysis of major volatiles by GC×GC-TOF-MS would require a rather different setup than that applied in this study, with much larger modulation time and hot pulse duration, not applicable for minor and trace compounds. Major volatile aroma compounds determined by GC-MS are listed in Table 1, grouped according to chemical class, and sorted within each class in order of decreasing F-ratio obtained by one-way ANOVA. Twenty-one monoterpenoids and a sesquiterpenoid trans-nerolidol, eight C13-norisoprenoids, two benzenoids, four alcohols, four acids, and 11 esters were quantified. Table S2 reports the concentrations of the identified volatile compounds in each of the investigated wines.
Table 1.
Concentrations (μg/L) of volatile aroma compounds found in Croatian monovarietal wines after headspace solid-phase microextraction followed by gas chromatography-mass spectrometry (HS-SPME/GC–MS) sorted by compound class and descending Fisher F-ratio.
| No. | Volatile Compounds | tR | ID | LRIexp | LRIlit | F-Ratio | Variety | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (min:s) | MI | PO | MA | KR | SK | ||||||
| Terpenes | |||||||||||
| 1 | α-Terpineol | 39:59 | S, MS, LRI | 1684 | 1684 | 35.07 | 15.65 ± 7.04 b | 10.92 ± 3.16 bc | 5.50 ± 2.10 cd | 1.98 ± 0.89 d | 40.49 ± 11.05 a |
| 2 | Monoterpene (n.i.; m/z 59, 93, 121) |
28:29 | MS | 1441 | - | 27.68 | 1.03 ± 0.50 b | 0.64 ± 0.24 bc | 0.27 ± 0.24 cd | 0.02 ± 0.04 d | 2.79 ± 1.01 a |
| 3 | Linalool | 33:10 | S, MS, LRI | 1542 | 1542 | 24.71 | 68.00 ± 27.76 b | 38.17 ± 8.05 c | 18.52 ± 8.28 d | 7.64 ± 2.56 d | 90.75 ± 15.48 a |
| 4 | Limonene | 15:17 | MS, LRI | 1191 | 1196 | 18.91 | 1.33 ± 0.68 b | 0.98 ± 0.28 b | 0.19 ± 0.05 c | 0.36 ± 0.11 c | 2.66 ± 1.01 a |
| 5 | Nerol | 44:35 | S, MS, LRI | 1791 | 1791 | 16.31 | 13.38 ± 6.72 a | 6.49 ± 1.96 b | 4.55 ± 1.58 bc | 1.06 ± 0.46 c | 17.36 ± 3.74 a |
| 6 | cis-Linalool furan oxide | 29:24 | MS, LRI | 1464 | 1464 | 12.57 | 0.08 ± 0.03 b | 0.18 ± 0.06 a | 0.06 ± 0.05 b | 0.02 ± 0.01 b | 0.20 ± 0.11 a |
| 7 | Monoterpenyl acetate (n.i.; m/z 93, 69, 121) |
21:55 | MS | 1302 | - | 12.50 | 3.12 ± 1.85 a | 1.11 ± 0.27 b | 0.59 ± 0.36 b | 0.12 ± 0.06 b | 3.16 ± 0.84 a |
| 8 | 4-Terpineol | 35:37 | MS, LRI | 1594 | 1596 | 11.60 | 0.24 ± 0.10 b | 0.24 ± 0.06 b | 0.23 ± 0.11 b | 0.11 ± 0.03 c | 0.51 ± 0.08 a |
| 9 | β-Pinene | 13:45 | MS, LRI | 1146 | 1145 | 11.46 | 4.40 ± 2.41 a | 2.43 ± 0.75 bc | 0.40 ± 0.15 d | 1.16 ± 0.37 cd | 4.17 ± 1.06 ab |
| 10 | Ho-Trienol | 36:02 | MS, LRI | 1601 | 1601 | 10.44 | 7.45 ± 3.95 a | 6.95 ± 2.01 ab | 1.71 ± 1.14 c | 1.60 ± 0.76 c | 4.18 ± 0.79 bc |
| 11 | trans-Rose oxide | 23:23 | MS, LRI | 1352 | 1341 | 10.32 | 0.27 ± 0.08 b | 0.21 ± 0.04 bc | 0.15 ± 0.07 c | 0.16 ± 0.03 bc | 0.59 ± 0.34 a |
| 12 | Monoterpene (n.i.; m/z 93, 69, 41) |
29:56 | MS | 1476 | - | 8.51 | 0.49 ± 0.28 b | 0.25 ± 0.07 c | 0.24 ± 0.17 c | 0.12 ± 0.13 c | 0.77 ± 0.24 a |
| 13 | trans-Ocimene | 18:03 | MS, LRI | 1252 | 1250 | 8.45 | 1.58 ± 0.92 a | 1.27 ± 0.41 ab | 0.17 ± 0.07 c | 0.55 ± 0.18 bc | 1.30 ± 0.50 ab |
| 14 | Citronellol | 43:11 | S, MS, LRI | 1758 | 1758 | 8.34 | 5.02 ± 0.61 a | 5.09 ± 0.69 a | 5.30 ± 1.78 a | 2.56 ± 0.30 b | 5.60 ± 1.75 a |
| 15 | Nerol oxide | 29:18 | MS, LRI | 1459 | 1464 | 7.01 | 3.04 ± 1.12 a | 3.74 ± 1.82 a | 1.35 ± 1.17 b | 1.11 ± 0.40 b | 4.11 ± 1.44 a |
| 16 | Geranyl acetone | 47:01 | MS, LRI | 1845 | 1845 | 5.38 | 2.93 ± 0.58 b | 3.58 ± 0.99 b | 7.58 ± 4.80 a | 2.64 ± 0.39 b | 2.55 ± 1.14 b |
| 17 | trans-Linalool pyran oxide | 41:49 | MS, LRI | 1726 | 1752 | 4.85 | 0.08 ± 0.02 b | 0.13 ± 0.05 a | 0.07 ± 0.05 b | 0.04 ± 0.03 b | 0.06 ± 0.02 b |
| 18 | trans-Nerolidol | 54:39 | MS, LRI | 2031 | 2031 | 4.61 | 2.89 ± 0.50 a | 3.17 ± 0.59 a | 2.66 ± 1.58 ab | 1.59 ± 0.22 b | 1.53 ± 0.35 b |
| 19 | Monoterpene (n.i.; m/z 121, 93, 136) |
31:30 | MS | 1509 | - | 3.09 | 2.45 ± 0.49 a | 2.41 ± 0.56 a | 2.11 ± 0.73 a | 1.11 ± 0.16 b | 2.88 ± 2.79 a |
| 20 | Geraniol | 46:35 | S, MS, LRI | 1838 | 1838 | 2.93 | 40.64 ± 21.59 ab | 24.23 ± 8.96 ab | 39.96 ± 48.27 ab | 2.73 ± 1.56 b | 46.19 ± 10.53 ab |
| 21 | Geranyl ethyl ether | 31:54 | MS, LRI | 1511 | 1499 | 2.69 | 0.53 ± 0.33 | 0.86 ± 0.97 | 1.08 ± 0.84 | 0.05 ± 0.02 | 0.82 ± 0.25 |
| 22 | α-Terpinolene | 19:34 | MS, LRI | 1287 | 1281 | 2.32 | 0.49 ± 0.29 | 0.73 ± 0.92 | 0.07 ± 0.04 | 0.14 ± 0.07 | 0.33 ± 0.26 |
| C13-norisoprenoids | |||||||||||
| 23 | Vitispirane II | 31:16 | MS, LRI | 1523 | 1529 | 9.85 | 0.07 ± 0.02 c | 0.34 ± 0.16 a | 0.20 ± 0.10 b | 0.09 ± 0.01 c | 0.14 ± 0.06 bc |
| 24 | β-Damascenone | 45:26 | MS, LRI | 1809 | 1809 | 7.09 | 3.52 ± 0.69 a | 2.81 ± 1.42 ab | 1.99 ± 0.58 bc | 2.28 ± 0.25 b | 0.89 ± 0.29 c |
| 25 | Actinidol I | 49:55 | MS, LRI | 1914 | 1914 | 5.59 | 0.12 ± 0.05 a | 0.16 ± 0.06 a | 0.13 ± 0.07 a | 0.04 ± 0.01 b | 0.09 ± 0.03 ab |
| 26 | Actinidol II | 50:27 | MS, LRI | 1927 | 1927 | 5.10 | 0.20 ± 0.08 a | 0.23 ± 0.07 a | 0.23 ± 0.10 a | 0.08 ± 0.01 b | 0.16 ± 0.04 ab |
| 27 | Vitispirane I | 32:08 | MS, LRI | 1521 | 1526 | 5.03 | 0.09 ± 0.04 c | 0.46 ± 0.24 a | 0.33 ± 0.24 ab | 0.19 ± 0.05 bc | 0.32 ± 0.18 abc |
| 28 | β-Ionone | 50:17 | S, MS, LRI | 1923 | 1923 | 3.89 | 0.06 ± 0.01 ab | 0.05 ± 0.01 b | 0.07 ± 0.01 a | 0.05 ± 0.01 b | 0.07 ± 0.01 a |
| 29 | Actinidol ethyl ether I | 40:25 | MS, LRI | 1690 | 1690 | 3.37 | 0.25 ± 0.12 bc | 0.43 ± 0.24 a | 0.34 ± 0.25 ab | 0.11 ± 0.02 c | 0.24 ± 0.06 bc |
| 30 | Actinidol ethyl ether II | 41:49 | MS, LRI | 1723 | 1723 | 2.76 | 0.15 ± 0.07 ab | 0.25 ± 0.16 a | 0.20 ± 0.15 a | 0.06 ± 0.01 b | 0.15 ± 0.04 ab |
| Benzenoids | |||||||||||
| 31 | Ethyl cinnamate | 57:33 | S, MS, LRI | 2111 | 2122 | 6.96 | 0.41 ± 0.19 b | 1.16 ± 0.78 a | 0.39 ± 0.08 b | 0.21 ± 0.10 b | 0.16 ± 0.10 b |
| 32 | Benzaldehyde | 31:26 | S, MS, LRI | 1508 | 1509 | 0.84 | 1.66 ± 1.25 | 3.48 ± 5.40 | 1.17 ± 0.54 | 2.56 ± 0.81 | 3.11 ± 1.57 |
| Alcohols | |||||||||||
| 33 | 1-Hexanol | 23:35 | S, MS, LRI | 1356 | 1357 | 25.56 | 792.14 ± 264.44 b | 949.93 ± 179.86 b | 859.15 ± 171.18 b | 321.89 ± 32.90 c | 1544.09 ± 146.31 a |
| 34 | cis-3-Hexen-1-ol | 25:03 | S, MS, LRI | 1379 | 1379 | 12.73 | 77.49 ± 40.64 c | 299.33 ± 113.23 a | 193.20 ± 123.23 b | 26.16 ± 4.63 c | 54.67 ± 23.77 c |
| 35 | 2-Phenylethanol | 48:52 | S, MS, LRI | 1891 | 1893 | 7.16 | 20,047.0 ± 4767.1 b | 33,176.1 ± 4679.3 a | 32,117.2 ± 10,870.7 a | 20,712.5 ± 6134.8 b | 17,665.9 ± 1061.0 b |
| 36 | trans-3-Hexen-1-ol | 24:03 | S, MS, LRI | 1361 | 1361 | 1.73 | 61.38 ± 24.09 | 45.64 ± 17.99 | 46.57 ± 10.28 | 43.09 ± 10.26 | 63.87 ± 22.80 |
| Acids | |||||||||||
| 37 | Decanoic acid | 62:49 | S, MS, LRI | 2257 | 2258 | 5.05 | 646.02 ± 179.70 b | 1627.60 ± 659.33 a | 1062.71 ± 505.33 b | 994.33 ± 67.19 b | 1090.36 ± 494.95 ab |
| 38 | Octanoic acid | 54:56 | S, MS, LRI | 2043 | 2042 | 4.03 | 4294.07 ± 796.78 b | 6239.74 ± 1532.91 a | 5147.23 ± 1562.12 ab | 6219.42 ± 455.69 a | 6359.73 ± 1152.33 a |
| 39 | Hexanoic acid | 46:10 | S, MS, LRI | 1830 | 1828 | 3.05 | 5715.09 ± 552.13 ab | 5184.65 ± 722.46 b | 5284.54 ± 1710.50 b | 6487.89 ± 603.01 a | 7025.45 ± 1103.35 a |
| 40 | Butyric acid | 36:28 | S, MS, LRI | 1612 | 1612 | 0.54 | 1766.10 ± 323.75 | 1607.09 ± 231.34 | 1685.41 ± 407.86 | 1581.53 ± 184.63 | 1788.32 ± 346.09 |
| Esters | |||||||||||
| 41 | 2-Phenethyl acetate | 45:03 | S, MS, LRI | 1803 | 1801 | 9.02 | 2230.06 ± 481.79 b | 4731.20 ± 1467.85 a | 2359.08 ± 1289.62 b | 2579.92 ± 287.25 b | 1750.70 ± 284.91 b |
| 42 | Ethyl octanoate | 28:06 | S, MS, LRI | 1435 | 1435 | 8.88 | 1211.04 ± 239.22 a | 1086.51 ± 223.88 a | 817.08 ± 231.10 b | 701.64 ± 160.66 b | 544.02 ± 243.59 b |
| 43 | Ethyl hexanoate | 17:35 | S, MS, LRI | 1236 | 1236 | 6.80 | 721.60 ± 172.38 a | 379.34 ± 86.89 c | 463.42 ± 153.50 bc | 580.60 ± 120.60 ab | 474.95 ± 108.08 bc |
| 44 | Hexyl acetate | 19:26 | S, MS, LRI | 1272 | 1272 | 6.10 | 216.64 ± 52.04 a | 204.45 ± 73.60 a | 123.25 ± 54.35 b | 107.91 ± 34.65 b | 207.09 ± 40.86 a |
| 45 | Ethyl decanoate | 37:43 | S, MS, LRI | 1637 | 1638 | 5.61 | 302.58 ± 46.92 a | 279.95 ± 69.24 ab | 179.10 ± 81.94 c | 220.08 ± 29.45 bc | 199.26 ± 29.89 bc |
| 46 | Isoamyl acetate | 12:29 | S, MS, LRI | 1120 | 1122 | 3.97 | 3299.12 ± 1092.74 a | 3321.37 ± 1674.71 a | 1460.92 ± 566.57 b | 2397.45 ± 774.95 ab | 1879.81 ± 562.20 ab |
| 47 | Ethyl butyrate | 09:27 | S, MS, LRI | 1030 | 1030 | 3.09 | 456.83 ± 69.21 a | 415.44 ± 50.58 ab | 363.29 ± 80.70 b | 367.30 ± 47.60 b | 350.99 ± 74.33 b |
| 48 | Ethyl 3-methylbutyrate | 10:31 | S, MS, LRI | 1065 | 1065 | 2.58 | 8.51 ± 1.98 | 14.79 ± 5.57 | 12.86 ± 5.26 | 11.34 ± 4.39 | 8.39 ± 0.88 |
| 49 | Ethyl 2-methylbutyrate | 10:00 | S, MS, LRI | 1049 | 1049 | 2.17 | 4.19 ± 1.13 | 6.57 ± 2.31 | 6.57 ± 2.70 | 6.25 ± 2.48 | 4.01 ± 0.62 |
| 50 | Diethyl succinate | 39:04 | S, MS, LRI | 1667 | 1669 | 1.53 | 1634.59 ± 398.33 | 1917.40 ± 1362.67 | 1665.03 ± 858.64 | 997.49 ± 290.90 | 1064.55 ± 106.44 |
| 51 | Ethyl lactate | 22:56 | S, MS, LRI | 1341 | 1341 | 0.58 | 25,943.4 ± 13,586.8 | 45,815.9 ± 55,981.7 | 34,462.1 ± 16,552.6 | 25,359.5 ± 12,701.8 | 32654.3 ± 7282.3 |
ID—identification of compounds; S—retention time and mass spectrum consistent with that of the pure standard and with NIST05 mass spectra electronic library; LRI—linear retention index consistent with that found in literature; MS—mass spectra consistent with that from NIST05 mass spectra electronic library or literature; n.i.—not identified. The compounds with only MS symbol in ID column were tentatively identified. The compounds for which pure standards were not available (without symbol S in the ID column) were quantified semi-quantitatively and their concentrations were expressed as equivalents of compounds with similar chemical structure assuming a response factor = 1. LRIexp—linear retention index obtained experimentally. Varieties: MI—Malvazija istarska, PO—Pošip, MA—Maraština, KR—Kraljevina, SK—Škrlet. Different superscript lowercase letters in a row represent statistically significant differences between mean values at p < 0.05 obtained by one-way ANOVA and least significant difference (LSD) test.
Among terpenes, major monoterpenols such as linalool, geraniol, α-terpineol, and nerol were found in the highest concentration, which was generally in agreement with previous findings on white wines [34,35,36]. The mentioned are among the most influential monoterpenoids to wine aroma, to which they significantly contribute with specific floral and fruity nuances due to their relatively low odor perception thresholds, such as, for example, 15 μg/L for linalool [35,37]. The highest F-ratio among all the compounds identified by GC-MS was determined for α-terpineol, followed by an unidentified monoterpene and linalool, confirming the importance of terpenes for wine varietal differentiation [35]. Many other (mono)terpenes also turned out to be important in this sense, while other compound classes exhibited lower F-ratios, with the exception of 1-hexanol. Such an outcome was expected to some extent, since terpenes are primary aroma compounds originating from grapes, both as free volatile molecules or released from glycosidic precursors. Their composition and amounts are genetically pre-determined: genetic variation in aroma biosynthesis genes cause differences in terpene concentrations between grapevine varieties. For example, a variant of 1-deoxy-D-xylulose-5-phosphate synthase, a gene responsible for the biosynthesis of terpenoids, causes pronounced increase in terpene concentration in Muscat and Gewürztraminer grapes, which gives wines of these varieties a recognizable floral aroma [4,38,39]. Monoterpenes are generally known to be responsible for varietal aroma of muscats and non-muscat aromatic varieties, such as Gewürtztraminer, Riesling, Müller-Thurgau, etc. [36,40,41], but were also found useful for the differentiation of wines of other, so-called semi-aromatic and neutral grape varieties [41,42,43,44,45]. Márquez, Castro, Natera, and García-Barroso [46] characterized the volatile fraction of Andalusian sweet wines made from Muscat and Pedro Ximenez varieties and, interestingly, also found that α-terpineol was the most powerful differentiator with the highest F-ratio, followed closely by linalool and limonene, similar as in this case.
In this study, the ratios of terpene concentrations in different monovarietal wines varied from compound to compound, but it was generally observed that wines from Škrlet, a relatively unexplored Croatian grape variety, were characterized by the highest concentrations of many important monoterpenes (Table 1), while the concentrations of other monoterpenes were also among the highest in the investigated wines. The concentrations of monoterpenes in Malvazija istarska wines were notable and generally in fair agreement with those reported previously for this variety, with linalool followed by geraniol as the most abundant [43,47,48,49]. Malvazija was followed by Pošip wine with intermediate concentrations, while Maraština and especially Kraljevina wines had the lowest terpene concentrations.
Although the content and composition of terpenes in grapes and wines is principally pre-determined by variety, they are susceptible to modulation in response to many factors, such as viticultural parameters including soil characteristics, exposure to sunlight, water status, defoliation, crop thinning, etc. [34,50], as well as pre-fermentation and fermentation practices and conditions [35,36]. Except the effect of variety, the differences between the investigated monovarietal wines were probably partly caused by different geographical origin (Istria, Dalmatia, continental Croatia), so the effects of variety and location probably acted in synergy. It is indeed known that low temperatures favor the production of aroma compounds in grapes [51], so it is possible that the highest concentration of monoterpenes in Škrlet wines from continental Croatia characterized by lower temperatures was at least partly due to the effect of climate. The same could be deduced for Malvazija wines coming from the northern, somewhat colder part of the Adriatic coast. Conversely, elevated temperatures have potential to reduce the aromatic potential of grapes [52], which is possibly a reason for somewhat lower concentrations of monoterpenes in Dalmatian Pošip and Maraština wines. Kraljevina wines, which had the lowest concentrations of terpenes despite originating from the continental part, could be an exception that confirms the rule.
C13-Norisoprenoids are also secondary metabolites in grapes, present in both aromatic and neutral varieties. They are formed as biodegradation products of carotenoid molecules, such as lutein, β-carotene, violaxanthin, and neoxanthin, via numerous formation mechanisms and intermediates during pre-fermentative steps, fermentation, and aging [53,54]. Four of them, β-damascenone, β-ionone, 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN), and trans-1-(2,3,6-trimethylphenyl)buta-1,3-diene (TPB), were commonly found in wine at concentrations surpassing their odor perception thresholds, meaning they can have a direct impact on wine aroma [34]. Especially important is β-damascenone with its pleasant odor reminiscent of honey, dried plum and stewed apple, and a very low perception threshold, which ranks it among the most important wine odorants [37]. β-Ionone, characterized by a threshold of the similar order of magnitude, also significantly contributes to wine aroma with an odor reminiscent of violets, while the contribution of TDN and TPB becomes relevant mostly in aged wines [34]. The concentrations of the majority of C13-norisoprenoids were generally higher in Dalmatian Pošip and Maraština, and the lowest in Kraljevina wines, although in particular cases with no statistical significance (Table 1). According to Marais and van Wyk [54] the concentration of β-damascenone is principally dependent on viticultural and winemaking conditions, while variety has less influence. Nevertheless, particular differences were observed: Malvazija wines were found to contain the highest concentration, although not different from that found in Pošip, while Škrlet had the lowest, not different from that found in Maraština wine. Malvazija was also characterized by the lowest concentration of vitispiranes together with Kraljevina wine. Among benzenoids, ethyl cinnamate emerged as a prominent marker of Pošip varietal origin, since it was found in the highest concentration in this wine.
C6-alcohols are formed mainly in pre-fermentation vinification steps by degradation of unsaturated fatty acids by the action of enzymes, as well as by liberation from glycosidic precursors. They may have an effect on wine aroma with their so-called green and herbal odors, but luckily have relatively high odor perception thresholds, such as 8000 μg/L for 1-hexanol [37], so only very high concentration can produce negative effects. Certain authors include C6-compounds among varietal aromas [16] and their concentrations were found useful in differentiation of particular wines based on variety [43,55]. The highest concentration of 1-hexanol was found in Škrlet, while Kraljevina contained the lowest amount (Table 1). Maraština, and especially Pošip wines were characterized by the highest concentration of unsaturated C6-alcohols. It is possible that the mentioned differences were a consequence of different enzymatic potentials and fatty acid precursor loads in grapes of these varieties [55].
Concentrations and the composition of fermentation aroma compounds are mainly affected by fermentation conditions, but may also be influenced by grape composition [56]. Many studies proved that the composition of volatile compounds formed in fermentation can be useful in differentiating wines of mostly neutral varieties equally or even more successful than by using, e.g., monoterpene concentrations [11,14,20,29]. This is more characteristic for C6–C10 fatty acids and the corresponding ethyl esters which, in contrast to acetates, are more dependent on the concentration of precursors and therefore on variety and conditions in vineyard, and less on the activity of yeast [57]. The average concentration of 2-phenylethanol was higher than the corresponding odor perception threshold of 10,000 μg/L in all the studied monovarietal wines, meaning this alcohol contributed significantly with its odor reminiscent of roses [37]. Pošip and Maraština had approximately 50% higher concentration of 2-phenylethanol in relation to the other investigated wines (Table 1). The concentrations of major volatile fatty acids (C6–C10) surpassed the corresponding odor perception thresholds of 420, 500, and 1000 μg/L, respectively [58], in all the investigated wines. Fatty acid production is determined in part by the initial composition of must [59] and therefore possibly by varietal origin. Malvazija istarska wines stood out with low concentrations of decanoic and octanoic acid. Among esters, Pošip was clearly differentiated from the other monovarietal wines by the highest concentration of 2-phenethyl acetate, which could have been related to the higher concentration of its precursor 2-phenylethanol found in this wine. However, it was stated previously that precursor concentrations do not significantly determine the concentrations of acetate esters formed by Saccharomyces cerevisiae, with the expression of alcohol acetyl transferase gene in yeast as a limiting factor [60]. Concentration of 2-phenethyl acetate in all the investigated wines was higher than the corresponding threshold of 250 μg/L [37], suggesting its floral odor participated in the aroma of all the wines. The major ethyl and acetate esters are among the most important volatile compounds for the fresh fruity aroma of young white wines to which they significantly contribute by commonly multiply surpassing their rather low odor perception thresholds, such as 30 μg/L for isoamyl acetate, 20 μg/L for ethyl butyrate, 5 μg/L for ethyl hexanoate, and 2 μg/L for ethyl octanoate [37]. The highest concentration of linear middle-chain ethyl esters and acetates other than 2-phenylethyl acetate, although in some cases without statistical significance, was noted in Malvazija istarska wines. Pošip was also relatively abundant in these esters, except for ethyl hexanoate which was found in the lowest concentration in this and in Maraština wines. Although hexanoic acid is mainly formed in fermentation, grapes also contain non-negligible concentration. This means that the concentration of ethyl hexanoate in wine is probably partly influenced by the concentration of its precursor, hexanoic acid, in grapes [4], so the lower concentration of ethyl hexanoate in Pošip and Maraština could have been influenced by a genotype.
3.2. GC×GC-TOF-MS
A characteristic HS-SPME/GC×GC-TOF-MS analysis 2D chromatogram of volatile compounds in Malvazija istarska wine is shown in Figure S1. It can be seen that many compounds which were separated by the second dimension column had the same retention times on the first, meaning these compounds would not be adequately separated by the conventional GC-MS. The average concentrations of volatile compounds (tentatively) identified in the investigated wines after GC×GC-TOF-MS analysis are reported in Table 2, while the concentrations found in each of the investigated wines are reported in Table S3. Compounds were grouped according to chemical class, and sorted within each class in order of decreasing F-ratio determined by one-way ANOVA. Three hundred and seventeen (317) volatile aroma compounds were identified, including 53 terpenes, 10 norisoprenoids, 50 benzenoids, 5 hydrocarbons, 7 aldehydes, 24 ketones, 32 alcohols, 16 acids, 73 esters, 5 volatile phenols, 17 furanoids and lactones, 19 sulfur containing compounds, and 6 other compounds. GC×GC-TOF-MS exhibited superior peak annotation ability than GC-MS which enabled the identification of a much larger number of compounds, as a consequence of higher separation efficiency, enhanced sensitivity, and clearer mass spectra allowed by separation on two different phases [23]. Other factors which could have affected the differences between the results obtained by the two techniques/methods were the absolute sensitivity of the analyzers, SPME conditions (sample volume and dilution, duration and temperature of extraction, fiber length, etc.), and others. To our knowledge, with 350 compounds identified by GC-MS and GC×GC-TOF-MS combined, this study reported one of the most detailed volatile aroma profiles in wine to date. It has to be noted that for particular compounds which were analyzed and reported by both the techniques applied the obtained absolute concentrations differed due to different quantification methods used: quantitative analysis with the use of standards solutions and calibration curves in GC-MS, and semi-quantification relative to internal standard 1-octanol concentration, assuming a response factor equal to one, in GC×GC-TOF-MS analysis, respectively. The concentrations of many volatile compounds were found to be significantly different between wines (161), but relatively few were found to be exclusive markers of particular variety.
Table 2.
Concentrations (μg/L relative to internal standard 2-octanol) of volatile aroma compounds found in Croatian monovarietal wines obtained by headspace solid-phase microextraction followed by comprehensive two-dimensional gas chromatography-mass spectrometry with time-of-flight mass spectrometric detection (HS-SPME/GC×GC-TOF-MS) sorted by compound class and descending Fisher F-ratio.
| No. | Volatile Compounds | tR (1D) | tR (2D) | ID | LRIexp | LRIlit | F-Ratio | Variety | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (min:s) | (min:s) | MI | PO | MA | KR | SK | ||||||
| Terpenes | ||||||||||||
| 1 | α-Terpineol | 18:47.2 | 00:01.2 | MS, LRI | 1710 | 1709 | 112.904 | 3.683 ± 1.391 b | 2.440 ± 0.424 c | 1.256 ± 0.455 d | 0.712 ± 0.369 d | 11.628 ± 0.495 a |
| 2 | Limonene | 08:01.0 | 00:01.8 | S, MS, LRI | 1191 | 1194 | 54.231 | 1.401 ± 0.746 b | 0.429 ± 0.261 c | 0.360 ± 0.105 c | 0.191 ± 0.114 c | 3.956 ± 0.291 a |
| 3 | Linalool | 15:50.0 | 00:01.1 | S, MS, LRI | 1541 | 1541 | 23.272 | 16.664 ± 7.072 b | 10.757 ± 1.712 c | 5.318 ± 1.567 d | 2.857 ± 0.970 d | 23.674 ± 3.494 a |
| 4 | trans-Alloocimene | 12:13.0 | 00:01.6 | MS, LRI | 1384 | 1388 | 22.080 | 0.362 ± 0.189 b | 0.137 ± 0.032 c | 0.088 ± 0.044 c | 0.040 ± 0.022 c | 0.753 ± 0.282 a |
| 5 | o-Cymene | 09:49.9 | 00:01.6 | S, MS, LRI | 1273 | 1268 | 20.745 | 1.305 ± 0.436 b | 0.631 ± 0.353 c | 0.441 ± 0.268 c | 0.278 ± 0.142 c | 2.613 ± 1.042 a |
| 6 | γ-Dehydro-ar-himachalene | 25:10.0 | 00:01.7 | MS | 2046 | - | 18.720 | 0.003 ± 0.003 b | 0.003 ± 0.003 b | 0.003 ± 0.002 b | 0.016 ± 0.006 a | 0.004 ± 0.004 b |
| 7 | β-Myrcene | 07:18.8 | 00:01.6 | S, MS, LRI | 1159 | 1159 | 17.244 | 2.351 ± 1.293 b | 0.885 ± 0.539 c | 0.331 ± 0.070 c | 0.183 ± 0.094 c | 4.353 ± 1.916 a |
| 8 | Nerol | 20:43.5 | 00:01.0 | S, MS, LRI | 1812 | 1811 | 16.944 | 0.568 ± 0.277 b | 0.220 ± 0.118 c | 0.225 ± 0.070 c | 0.077 ± 0.038 c | 0.810 ± 0.139 a |
| 9 | cis-α-Ocimene | 09:24.8 | 00:01.6 | MS, LRI | 1254 | 1255 | 14.724 | 2.278 ± 0.937 a | 0.754 ± 0.205 b | 0.459 ± 0.087 b | 0.337 ± 0.170 b | 3.070 ± 1.903 a |
| 10 | trans-Linalool furan oxide | 13:39.9 | 00:01.2 | S, MS, LRI | 1445 | 1450 | 14.601 | 0.444 ± 0.117 b | 0.480 ± 0.160 b | 0.221 ± 0.144 c | 0.157 ± 0.138 c | 0.872 ± 0.264 a |
| 11 | Ho-trienol | 17:07.0 | 00:01.0 | MS, LRI | 1607 | 1612 | 13.945 | 2.843 ± 1.206 a | 2.307 ± 0.568 a | 0.669 ± 0.405 b | 0.712 ± 0.395 b | 2.510 ± 0.219 a |
| 12 | Geraniol | 21:33.0 | 00:01.0 | S, MS, LRI | 1856 | 1857 | 11.761 | 0.432 ± 0.254 a | 0.225 ± 0.091 b | 0.124 ± 0.041 b | 0.084 ± 0.020 b | 0.589 ± 0.079 a |
| 13 | Carvomenthenal | 17:14.0 | 00:01.5 | MS, LRI | 1615 | 1629 | 11.321 | 0.381 ± 0.215 a | 0.193 ± 0.088 b | 0.142 ± 0.052 b | 0.057 ± 0.034 b | 0.525 ± 0.132 a |
| 14 | Linalool ethyl ether | 11:02.8 | 00:01.9 | MS, LRI | 1329 | 1331 | 10.900 | 5.419 ± 2.787 a | 1.744 ± 0.925 b | 0.998 ± 0.204 b | 0.444 ± 0.219 b | 6.365 ± 4.476 a |
| 15 | 4-Terpineol | 17:00.0 | 00:01.3 | S, MS, LRI | 1600 | 1597 | 10.895 | 0.464 ± 0.097 b | 0.363 ± 0.069 cd | 0.418 ± 0.116 bc | 0.289 ± 0.083 d | 0.676 ± 0.045 a |
| 16 | α-Terpinolene | 10:03.7 | 00:01.9 | S, MS, LRI | 1284 | 1282 | 10.846 | 1.630 ± 0.872 b | 0.520 ± 0.288 c | 0.387 ± 0.217 c | 0.173 ± 0.085 c | 3.391 ± 2.459 a |
| 17 | Geranyl ethyl ether | 15:08.0 | 00:01.8 | MS, LRI | 1506 | 1506 | 10.391 | 2.042 ± 0.965 a | 0.710 ± 0.328 b | 0.500 ± 0.186 b | 0.222 ± 0.161 b | 2.960 ± 2.199 a |
| 18 | Monoterpene (n.i.; m/z 93, 121, 136) | 13:54.1 | 00:01.9 | MS | 1455 | - | 9.899 | 10.628 ± 4.686 a | 4.420 ± 1.974 b | 2.737 ± 1.576 b | 1.104 ± 0.702 b | 16.227 ± 12.511 a |
| 19 | Neryl ethyl ether | 14:26.5 | 00:01.8 | MS, LRI | 1477 | 1468 | 8.918 | 0.334 ± 0.175 b | 0.143 ± 0.065 bc | 0.100 ± 0.067 bc | 0.023 ± 0.021 c | 0.863 ± 0.729 a |
| 20 | Monoterpene (n.i.; m/z 68, 93, 121) | 08:15.2 | 00:01.8 | MS | 1202 | - | 8.901 | 0.252 ± 0.272 b | 0.126 ± 0.077 bc | 0.047± 0.021 c | 0.020 ± 0.015 c | 0.575 ± 0.180 a |
| 21 | p-Cymenene | 13:30.2 | 00:01.5 | MS, LRI | 1439 | 1438 | 7.409 | 1.332 ± 0.236 a | 0.899 ± 0.190 b | 0.918 ± 0.307 b | 0.733 ± 0.210 b | 1.711 ± 0.786 a |
| 22 | trans-β-Ocimene | 09:01.7 | 00:01.7 | S, MS, LRI | 1237 | 1241 | 6.833 | 0.078 ± 0.148 b | 0.067 ± 0.087 b | 0.099 ± 0.109 b | 0.014 ± 0.019 b | 0.919 ± 0.926 a |
| 23 | β-Calacorene | 22:43.0 | 00:01.8 | MS, LRI | 1919 | 1918 | 5.495 | 0.108 ± 0.018 ab | 0.075 ± 0.011 c | 0.078 ± 0.011 c | 0.131 ± 0.044 a | 0.072 ± 0.052 c |
| 24 | cis-Z-α-Bisabolene epoxide | 24:28.0 | 00:01.3 | MS, LRI | 2010 | 2007 | 5.329 | 0.002 ± 0.003 c | 0.008 ± 0.004 bc | 0.010 ± 0.009 b | 0.013 ± 0.006 ab | 0.023 ± 0.016 a |
| 25 | trans-Linalool pyran oxide | 19:34.0 | 00:01.1 | MS, LRI | 1751 | 1752 | 4.346 | 0.059 ± 0.018 ab | 0.082 ± 0.049 a | 0.029 ± 0.031 b | 0.031 ± 0.019 b | 0.086 ± 0.025 a |
| 26 | Cadalene | 27:46.2 | 00:01.6 | MS, LRI | >2100 | 2191 | 3.956 | 0.047 ± 0.010 b | 0.039 ± 0.017 b | 0.035 ± 0.011 b | 0.064 ± 0.022 a | 0.032 ± 0.021 b |
| 27 | Isogeraniol | 20:55.4 | 00:01.0 | MS, LRI | 1822 | 1828 | 3.457 | 0.036 ± 0.030 a | 0.018 ± 0.014 ab | 0.005 ± 0.006 b | 0.010 ± 0.011 b | 0.015 ± 0.007 ab |
| 28 | α-Terpinene | 07:33.6 | 00:01.8 | S, MS, LRI | 1170 | 1175 | 3.225 | 0.033 ± 0.061 b | 0.006 ± 0.010 b | 0.006 ± 0.010 b | 0.007 ± 0.015 b | 0.106 ± 0.120 a |
| 29 | Sesquiterpene (n.i.; m/z 119, 93, 69) | 19:48.0 | 00:01.8 | MS | 1763 | - | 2.809 | 0.064 ± 0.010 a | 0.038 ± 0.020 b | 0.040 ± 0.014 b | 0.045 ± 0.012 b | 0.042 ± 0.038 b |
| 30 | Menthol | 17:42.0 | 00:01.1 | MS, LRI | 1644 | 1641 | 2.746 | 0.129 ± 0.050 b | 0.222 ± 0.283 b | 0.115 ± 0.032 b | 0.099 ± 0.023 b | 1.177 ± 1.847 a |
| 31 | Citronellol | 20:02.9 | 00:01.1 | S, MS, LRI | 1776 | 1777 | 2.724 | 0.303 ± 0.037 ab | 0.250 ± 0.089 ab | 0.260 ± 0.089 ab | 0.154 ± 0.074 b | 0.309 ± 0.229 ab |
| 32 | α-Farnesene II | 19:48.0 | 00:01.9 | S, MS, LRI | 1763 | 1762 | 2.118 | 0.053 ± 0.018 | 0.029 ± 0.016 | 0.026 ± 0.023 | 0.039 ± 0.021 | 0.047 ± 0.026 |
| 33 | Monoterpene (n.i.; m/z 93, 121, 94) | 20:48.1 | 00:01.7 | MS | 1816 | - | 2.054 | 0.056 ± 0.034 | 0.067 ± 0.027 | 0.041 ± 0.041 | 0.028 ± 0.015 | 0.028 ± 0.009 |
| 34 | γ-Cadinene | 19:55.0 | 00:02.1 | MS, LRI | 1769 | 1774 | 1.895 | 0.020 ± 0.003 | 0.013 ± 0.007 | 0.016 ± 0.004 | 0.014 ± 0.007 | 0.012 ± 0.011 |
| 35 | (+)-Cuparene | 21:05.0 | 00:02.0 | MS, LRI | 1831 | 1830 | 1.847 | 0.020 ± 0.001 | 0.014 ± 0.010 | 0.017 ± 0.004 | 0.012 ± 0.007 | 0.012 ± 0.010 |
| 36 | trans-Calamenene | 21:19.0 | 00:01.9 | MS, LRI | 1844 | 1837 | 1.780 | 0.063 ± 0.019 | 0.056 ± 0.020 | 0.049 ± 0.011 | 0.077 ± 0.028 | 0.056 ± 0.023 |
| 37 | Citronellyl acetate | 18:10.0 | 00:01.5 | MS, LRI | 1673 | 1668 | 1.652 | 0.056 ± 0.031 | 0.082 ± 0.059 | 0.041 ± 0.022 | 0.035 ± 0.017 | 0.063 ± 0.060 |
| 38 | α-Calacorene | 22:29.0 | 00:01.8 | MS, LRI | 1906 | 1916 | 1.533 | 0.020 ± 0.003 | 0.014 ± 0.008 | 0.015 ± 0.005 | 0.019 ± 0.009 | 0.012 ± 0.010 |
| 39 | Dehydroaromadendrene | 28:20.9 | 00:01.4 | MS | >2100 | - | 1.306 | 0.001 ± 0.002 | 0.014 ± 0.036 | 0.001 ± 0.002 | 0.012 ± 0.015 | 0.028 ± 0.034 |
| 40 | Terpene (n.i.; m/z 121, 93, 136) | 15:08.0 | 00:01.8 | MS | 1506 | - | 1.276 | 0.891 ± 0.202 | 0.784 ± 0.328 | 0.788 ± 0.357 | 0.458 ± 0.172 | 0.836 ± 1.082 |
| 41 | β-Cyclocitral | 17:21.0 | 00:01.5 | S, MS, LRI | 1622 | 1629 | 1.195 | 0.067 ± 0.016 | 0.071 ± 0.017 | 0.066 ± 0.030 | 0.051 ± 0.014 | 0.052 ± 0.028 |
| 42 | α-Farnesene I | 18:17.0 | 00:01.9 | S, MS, LRI | 1681 | 1697 | 1.124 | 0.126 ± 0.043 | 0.074 ± 0.034 | 0.102 ± 0.058 | 0.115 ± 0.061 | 0.113 ± 0.046 |
| 43 | 2-Acetyl-2-carene | 23:11.0 | 00:01.3 | MS | 1943 | - | 1.088 | 0.030 ± 0.048 | 0.037 ± 0.039 | 0.047 ± 0.050 | 0.010 ± 0.013 | 0.054 ± 0.013 |
| 44 | γ-Isogeraniol | 20:29.2 | 00:01.0 | MS, LRI | 1799 | 1800 | 1.071 | 0.148 ± 0.134 | 0.123 ± 0.083 | 0.087 ± 0.064 | 0.061 ± 0.046 | 0.087 ± 0.078 |
| 45 | Cosmene | 13:41.6 | 00:01.4 | MS, LRI | 1446 | 1460 | 0.990 | 0.109 ± 0.117 | 0.056 ± 0.056 | 0.106 ± 0.068 | 0.080 ± 0.054 | 0.027 ± 0.011 |
| 46 | α-Curcumene | 20:16.0 | 00:01.8 | MS, LRI | 1787 | 1782 | 0.736 | 0.026 ± 0.014 | 0.019 ± 0.007 | 0.021 ± 0.007 | 0.029 ± 0.017 | 0.022 ± 0.011 |
| 47 | trans-Geranyl acetone | 21:40.0 | 00:01.5 | MS, LRI | 1863 | 1868 | 0.734 | 0.409 ± 0.437 | 0.228 ± 0.071 | 0.206 ± 0.060 | 0.422 ± 0.568 | 0.152 ± 0.036 |
| 48 | α-Bergamotene | 16:18.0 | 00:02.4 | MS, LRI | 1565 | 1585 | 0.675 | 0.027 ± 0.011 | 0.037 ± 0.027 | 0.058 ± 0.071 | 0.041 ± 0.020 | 0.043 ± 0.024 |
| 49 | Sesquiterpene (n.i.; m/z 93, 80, 121) | 20:16.0 | 00:02.0 | MS | 1787 | - | 0.596 | 0.021 ± 0.007 | 0.014 ± 0.008 | 0.020 ± 0.013 | 0.022 ± 0.014 | 0.022 ± 0.021 |
| 50 | Neryl acetate | 19:20.7 | 00:01.5 | MS, LRI | 1739 | 1742 | 0.576 | 0.085 ± 0.021 | 0.060 ± 0.050 | 0.058 ± 0.038 | 0.078 ± 0.054 | 0.092 ± 0.083 |
| 51 | 4-Thujanol | 15:20.1 | 00:01.7 | MS | 1516 | - | 0.444 | 0.055 ± 0.040 | 0.051 ± 0.029 | 0.067 ± 0.043 | 0.068 ± 0.024 | 0.046 ± 0.012 |
| 52 | Nerolidol | 24:56.2 | 00:01.3 | S, MS, LRI | 2034 | 2034 | 0.434 | 0.114 ± 0.049 | 0.102 ± 0.040 | 0.124 ± 0.087 | 0.142 ± 0.058 | 0.131 ± 0.064 |
| 53 | Geranyl acetate | 19:55.0 | 00:01.5 | S, MS, LRI | 1769 | 1768 | 0.413 | 0.037 ± 0.015 | 0.048 ± 0.038 | 0.035 ± 0.018 | 0.034 ± 0.013 | 0.034 ± 0.033 |
| C13-norisoprenoids | ||||||||||||
| 54 | 1,2-Dihydro-1,4,6-trimethylnaphthalene | 25:38.3 | 00:01.5 | MS | 2071 | - | 7.148 | 0.001 ± 0.001 b | 0.000 ± 0.000 b | 0.009 ± 0.008 b | 0.023 ± 0.018 a | 0.002 ± 0.001 b |
| 55 | β-Damascenone | 21:05.0 | 00:01.5 | S, MS, LRI | 1831 | 1832 | 6.736 | 8.245 ± 2.169 a | 5.770 ± 2.963 bc | 4.338 ± 1.563 cd | 6.981 ± 1.067 ab | 2.336 ± 0.303 d |
| 56 | α-Ionene | 14:28.9 | 00:02.0 | MS | 1479 | - | 5.379 | 0.045 ± 0.017 bc | 0.017 ± 0.023 c | 0.069 ± 0.047 ab | 0.085 ± 0.031 a | 0.032 ± 0.017 bc |
| 57 | Norisoprenoid (n.i.; m/z 69, 121, 105) | 20:00.5 | 00:01.6 | MS | 1774 | - | 5.061 | 0.248 ± 0.076 a | 0.163 ± 0.101 b | 0.127 ± 0.060 bc | 0.202 ± 0.058 ab | 0.055 ± 0.025 c |
| 58 | 3,4-Dehydro-β-ionone | 18:38.0 | 00:01.8 | MS | 1702 | - | 4.920 | 0.032 ± 0.009 a | 0.011 ± 0.010 b | 0.036 ± 0.017 a | 0.040 ± 0.020 a | 0.020 ± 0.004 ab |
| 59 | Vitispirane I | 15:29.0 | 00:01.9 | MS, LRI | 1524 | 1524 | 3.470 | 1.155 ± 0.481 c | 3.501 ± 1.555 ab | 4.058 ± 2.588 a | 2.200 ± 0.966 bc | 3.007 ± 2.552 abc |
| 60 | 1,2-Dihydro-1,5,8-trimethylnaphthalene | 19:41.0 | 00:01.6 | MS | 1757 | - | 2.579 | 0.360 ± 0.109 | 0.363 ± 0.186 | 0.682 ± 0.399 | 0.770 ± 0.451 | 1.085 ± 1.062 |
| 61 | Actinidol ethyl ether II | 18:52.0 | 00:01.9 | MS, LRI | 1714 | 1723 | 2.179 | 0.099 ± 0.082 | 0.181 ± 0.106 | 0.167 ± 0.130 | 0.055 ± 0.030 | 0.133 ± 0.067 |
| 62 | 1,2-Dihydro-1,1,6-trimethylnaphthalene (TDN) | 19:06.0 | 00:01.6 | S, MS, LRI | 1727 | 1729 | 0.885 | 0.021 ± 0.012 | 0.019 ± 0.010 | 0.031 ± 0.021 | 0.026 ± 0.018 | 0.015 ± 0.015 |
| 63 | trans-1-(2,3,6-Trimethylphenyl)buta-1,3-diene (TPB) | 21:12.0 | 00:01.5 | MS, LRI | 1837 | 1832 | 0.433 | 0.065 ± 0.029 | 0.057 ± 0.036 | 0.076 ± 0.048 | 0.057 ± 0.031 | 0.050 ± 0.025 |
| Benzenoids | ||||||||||||
| 64 | Ethyl benzoate | 18:17.0 | 00:01.2 | MS, LRI | 1681 | 1678 | 20.194 | 0.759 ± 0.232 b | 1.493 ± 0.366 a | 0.653 ± 0.128 b | 0.533 ± 0.118 b | 0.570 ± 0.115 b |
| 65 | 1,1’-Oxybisbenzene | 24:20.1 | 00:01.3 | MS, LRI | 2003 | 2017 | 18.956 | 0.011 ± 0.002 a | 0.004 ± 0.002 b | 0.004 ± 0.003 b | 0.003 ± 0.001 b | 0.003 ± 0.001 b |
| 66 | 2,3-Dihydro-1,1,5,6-tetramethyl-1H-indene I | 18:17.0 | 00:01.7 | MS | 1681 | - | 9.842 | 0.076 ± 0.019 bc | 0.027 ± 0.030 d | 0.105 ± 0.039 ab | 0.139 ± 0.054 a | 0.050 ± 0.013 cd |
| 67 | Octylbenzene | 19:27.0 | 00:01.8 | MS, LRI | 1745 | 1746 | 8.638 | 0.109 ± 0.011 a | 0.065 ± 0.022 b | 0.074 ± 0.010 b | 0.072 ± 0.012 b | 0.070 ± 0.032 b |
| 68 | trans-Edulan | 17:07.0 | 00:01.8 | MS, LRI | 1607 | 1602 | 7.938 | 0.039 ± 0.016 b | 0.084 ± 0.018 a | 0.056 ± 0.019 b | 0.042 ± 0.010 b | 0.035 ± 0.031 b |
| 69 | 2,3-Dihydro-1,1,5,6-tetramethyl-1H-indene II | 17:28.0 | 00:01.7 | MS | 1629 | - | 7.671 | 0.023 ± 0.006 bc | 0.007 ± 0.010 c | 0.038 ± 0.020 ab | 0.056 ± 0.030 a | 0.016 ± 0.004 bc |
| 70 | 3-Methylphenylacetylene | 14:33.0 | 00:01.3 | MS, LRI | 1481 | 1450.9 | 7.438 | 0.012 ± 0.002 b | 0.016 ± 0.006 b | 0.013 ± 0.007 b | 0.032 ± 0.013 a | 0.028 ± 0.012 a |
| 71 | Benzoic acid | 30:48.9 | 00:00.8 | S, MS, LRI | >2100 | 2438 | 6.952 | 0.269 ± 0.021 bc | 0.412 ± 0.064 a | 0.350 ± 0.047 a | 0.233 ± 0.107 c | 0.334 ± 0.111 ab |
| 72 | Azulene | 19:34.0 | 00:01.3 | MS, LRI | 1751 | 1746 | 6.891 | 0.240 ± 0.058 a | 0.181 ± 0.044 b | 0.165 ± 0.042 bc | 0.130 ± 0.039 c | 0.117 ± 0.039 c |
| 73 | Trimethyl-tetrahydronaphthalene | 13:12.1 | 00:01.8 | MS | 1426 | - | 6.830 | 0.007 ± 0.012 c | 0.002 ± 0.004 c | 0.056 ± 0.056 ab | 0.102 ± 0.070 a | 0.007 ± 0.008 c |
| 74 | Benzeneacetaldehyde | 17:49.0 | 00:01.1 | S, MS, LRI | 1651 | 1648 | 6.827 | 5.920 ± 1.513 c | 10.269 ± 2.174 a | 8.487 ± 2.065 ab | 5.884 ± 2.053 c | 6.501 ± 1.630 bc |
| 75 | m-Methoxyanisole | 19:48.0 | 00:01.2 | MS, LRI | 1763 | 1761 | 6.715 | 0.009 ± 0.013 c | 0.035 ± 0.021 b | 0.009 ± 0.011 c | 0.013 ± 0.013 bc | 0.070 ± 0.052 a |
| 76 | Benzenoid (n.i.; m/z 115, 130, 129) | 16:37.2 | 00:01.4 | MS | 1581 | - | 5.764 | 0.007 ± 0.003 b | 0.005 ± 0.004 b | 0.005 ± 0.004 b | 0.014 ± 0.005 a | 0.009 ± 0.003 ab |
| 77 | 6-[1-(Hydroxymethyl)vinyl]-4,8a-dimethyl-1,2,4a,5,6,7,8,8a-octahydro-2-naphthalenol | 19:54.7 | 00:02.0 | MS | 1769 | - | 5.587 | 0.010 ± 0.014 b | 0.010 ± 0.011 b | 0.009 ± 0.010 b | 0.041 ± 0.023 a | 0.013 ± 0.011 b |
| 78 | Prehnitene | 14:40.0 | 00:01.5 | MS, LRI | 1486 | 1476 | 5.516 | 0.198 ± 0.035 ab | 0.160 ± 0.052 bc | 0.249 ± 0.080 a | 0.124 ± 0.034 c | 0.124 ± 0.089 c |
| 79 | 2-Methylnaphthalene | 22:15.0 | 00:01.3 | MS, LRI | 1894 | 1872 | 4.321 | 0.021 ± 0.004 a | 0.015 ± 0.003 b | 0.014 ± 0.004 b | 0.015 ± 0.005 b | 0.012 ± 0.003 b |
| 80 | meso-2,3-Diphenylbutane | 17:07.0 | 00:01.3 | MS | 1607 | - | 4.291 | 0.025 ± 0.017 b | 0.050 ± 0.016 a | 0.037 ± 0.009 ab | 0.027 ± 0.010 b | 0.032 ± 0.007 ab |
| 81 | 4-Ethylbenzaldehyde | 19:34.0 | 00:01.2 | MS, LRI | 1751 | 1747 | 4.168 | 0.059 ± 0.010 ab | 0.067 ± 0.014 a | 0.060 ± 0.015 ab | 0.043 ± 0.010 c | 0.048 ± 0.008 bc |
| 82 | Styrene | 09:27.4 | 00:05.0 | MS, LRI | 1256 | 1257 | 3.578 | 2.067 ± 0.516 ab | 2.462 ± 0.859 a | 2.161 ± 0.275 ab | 1.701 ± 0.419 bc | 1.223 ± 0.083 c |
| 83 | Ethyl o-methylbenzoate | 19:34.2 | 00:01.3 | MS | 1751 | - | 3.148 | 0.039 ± 0.005 ab | 0.041 ± 0.005 a | 0.034 ± 0.005 abc | 0.028 ± 0.013 c | 0.027 ± 0.015 c |
| 84 | 2,3-Dihydrobenzofuran | 16:46.0 | 00:01.2 | MS | 1588 | - | 3.122 | 0.025 ± 0.011 b | 0.046 ± 0.014 a | 0.039 ± 0.013 ab | 0.029 ± 0.014 b | 0.029 ± 0.001 b |
| 85 | Benzofuran | 15:01.0 | 00:01.1 | MS, LRI | 1500 | 1496 | 3.121 | 0.040 ± 0.013 b | 0.061 ± 0.022 a | 0.047 ± 0.015 ab | 0.040 ± 0.013 b | 0.030 ± 0.005 b |
| 86 | Benzonitrile | 17:00.0 | 00:01.0 | MS, LRI | 1600 | 1591 | 3.041 | 0.033 ± 0.015 b | 0.064 ± 0.026 a | 0.052 ± 0.021 ab | 0.038 ± 0.016 b | 0.039 ± 0.005 ab |
| 87 | α,α-Dimethylbenzenemethanol | 19:55.2 | 00:01.0 | MS, LRI | 1769 | 1770 | 2.981 | 0.021 ± 0.009 b | 0.033 ± 0.018 b | 0.024 ± 0.008 b | 0.027 ± 0.007 b | 0.203 ± 0.309 a |
| 88 | Methyl 2-(benzyloxy)propanoate | 23:18.0 | 00:01.3 | MS | 1949 | - | 2.958 | 0.825 ± 0.861 a | 0.144 ± 0.151 b | 0.107 ± 0.138 b | 0.211 ± 0.456 b | 0.018 ± 0.017 b |
| 89 | α-Methylstyrene | 11:10.8 | 00:01.3 | MS, LRI | 1336 | 1325 | 2.727 | 0.006 ± 0.007 | 0.014 ± 0.006 | 0.015 ± 0.007 | 0.017 ± 0.008 | 0.118 ± 0.192 |
| 90 | 3-Ethylbenzaldehyde | 18:59.4 | 00:01.2 | MS, LRI | 1721 | 1732 | 2.631 | 0.088 ± 0.013 | 0.092 ± 0.016 | 0.086 ± 0.023 | 0.065 ± 0.021 | 0.075 ± 0.004 |
| 91 | 3-Methylbenzofuran | 21:19.0 | 00:01.1 | MS | 1844 | - | 2.594 | 0.027 ± 0.006 | 0.046 ± 0.012 | 0.034 ± 0.016 | 0.032 ± 0.014 | 0.034 ± 0.006 |
| 92 | Styralyl isobutyrate | 23:31.8 | 00:01.4 | MS | 1961 | - | 2.462 | 0.065 ± 0.022 | 0.191 ± 0.121 | 0.166 ± 0.115 | 0.149 ± 0.069 | 0.097 ± 0.023 |
| 93 | 2’,5’-Dimethylcrotonophenone | 24:00.0 | 00:01.3 | MS | 1985 | - | 2.362 | 0.045 ± 0.017 | 0.040 ± 0.029 | 0.026 ± 0.019 | 0.040 ± 0.011 | 0.010 ± 0.009 |
| 94 | Ethyl benzenepropanoate | 22:15.0 | 00:01.3 | MS, LRI | 1894 | 1892 | 2.328 | 0.404 ± 0.256 | 0.509 ± 0.353 | 0.314 ± 0.180 | 0.206 ± 0.052 | 0.131 ± 0.079 |
| 95 | 1-Methylnapthalene | 21:40.0 | 00:01.3 | MS, LRI | 1863 | 1878 | 2.319 | 0.017 ± 0.005 | 0.016 ± 0.007 | 0.014 ± 0.003 | 0.011 ± 0.003 | 0.010 ± 0.001 |
| 96 | Ethyl salicylate | 21:35.8 | 00:01.1 | S, MS, LRI | 1859 | 1837 | 2.288 | 0.037 ± 0.054 | 0.005 ± 0.008 | 0.027 ± 0.049 | 0.011 ± 0.012 | 0.177 ± 0.296 |
| 97 | Methyl salicylate | 20:16.0 | 00:01.2 | S, MS, LRI | 1787 | 1789 | 2.151 | 3.457 ± 1.576 | 4.548 ± 6.357 | 2.447 ± 1.435 | 1.979 ± 0.985 | 14.629 ± 21.656 |
| 98 | 2-Methylbenzaldehyde | 17:27.8 | 00:01.1 | MS, LRI | 1629 | 1622 | 2.145 | 0.041 ± 0.014 | 0.057 ± 0.023 | 0.034 ± 0.023 | 0.032 ± 0.012 | 0.051 ± 0.025 |
| 99 | Durene | 13:16.0 | 00:01.5 | MS, LRI | 1429 | 1435 | 2.038 | 0.085 ± 0.033 | 0.084 ± 0.040 | 0.080 ± 0.027 | 0.056 ± 0.034 | 0.034 ± 0.028 |
| 100 | Butylated hydroxytoluene | 22:43.0 | 00:01.5 | MS, LRI | 1919 | 1920 | 1.558 | 0.314 ± 0.073 | 0.351 ± 0.163 | 0.295 ± 0.107 | 0.228 ± 0.067 | 0.210 ± 0.146 |
| 101 | Ethyl benzeneacetate | 20:30.0 | 00:01.2 | MS, LRI | 1799 | 1788 | 1.474 | 1.334 ± 0.310 | 3.173 ± 0.863 | 2.503 ± 1.245 | 3.294 ± 3.186 | 3.112 ± 2.566 |
| 102 | p-Methoxyanisole | 19:34.2 | 00:01.2 | MS, LRI | 1751 | 1752 | 1.309 | 0.153 ± 0.041 | 0.184 ± 0.074 | 0.141 ± 0.044 | 0.197 ± 0.052 | 0.191 ± 0.075 |
| 103 | Benzeneacetic acid | 32:45.2 | 00:00.8 | MS, LRI | >2100 | 2519 | 1.243 | 0.002 ± 0.000 | 0.005 ± 0.005 | 0.008 ± 0.007 | 0.004 ± 0.007 | 0.009 ± 0.013 |
| 104 | Benzyl alcohol | 22:01.0 | 00:00.9 | S, MS, LRI | 1881 | 1877 | 1.243 | 0.914 ± 1.413 | 2.007 ± 2.953 | 0.468 ± 0.243 | 0.354 ± 0.079 | 0.565 ± 0.297 |
| 105 | 2-Hydroxybenzeneacetic acid | 23:11.0 | 00:01.0 | MS | 1943 | - | 1.238 | 0.003 ± 0.008 | 0.008 ± 0.004 | 0.003 ± 0.003 | 0.005 ± 0.001 | 0.005 ± 0.002 |
| 106 | 2-Ethyl-m-xylene | 11:58.5 | 00:01.6 | MS, LRI | 1373 | 1372 | 1.224 | 0.106 ± 0.064 | 0.112 ± 0.059 | 0.082 ± 0.043 | 0.094 ± 0.040 | 0.040 ± 0.035 |
| 107 | Benzaldehyde | 15:16.8 | 00:05.8 | S, MS, LRI | 1514 | 1509 | 1.094 | 1.935 ± 0.978 | 4.662 ± 6.475 | 1.479 ± 0.377 | 2.161 ± 0.535 | 2.934 ± 2.144 |
| 108 | 2-(1,1-Dimethylethyl)-1,4-dimethoxybenzene | 22:29.0 | 00:01.4 | MS, LRI | 1906 | 1870 | 0.996 | 0.069 ± 0.010 | 0.061 ± 0.014 | 0.066 ± 0.017 | 0.075 ± 0.021 | 0.051 ± 0.044 |
| 109 | trans-1,2-Diphenylcyclobutane | 20:58.0 | 00:01.3 | MS | 1825 | - | 0.674 | 0.003 ± 0.002 | 0.003 ± 0.002 | 0.004 ± 0.007 | 0.002 ± 0.003 | 0.000 ± 0.000 |
| 110 | 3,3-Dimethoxy-1-phenylpropan-1-one | 09:56.8 | 00:05.6 | MS | 1278 | - | 0.569 | 0.037 ± 0.032 | 0.048 ± 0.054 | 0.052 ± 0.059 | 0.025 ± 0.027 | 0.062 ± 0.031 |
| 111 | trans-Anethole | 21:12.0 | 00:01.3 | S, MS, LRI | 1837 | 1834 | 0.502 | 0.423 ± 0.223 | 0.449 ± 0.199 | 0.390 ± 0.213 | 0.395 ± 0.309 | 0.613 ± 0.344 |
| 112 | cis-Anethole | 19:55.0 | 00:01.3 | S, MS, LRI | 1769 | 1780 | 0.322 | 0.013 ± 0.005 | 0.015 ± 0.007 | 0.014 ± 0.004 | 0.014 ± 0.007 | 0.017 ± 0.010 |
| 113 | 1,2-Dimethylbenzene | 07:41.0 | 00:05.1 | MS, LRI | 1176 | 1175 | 0.176 | 0.468 ± 0.333 | 0.412 ± 0.402 | 0.503 ± 0.355 | 0.356 ± 0.312 | 0.432 ± 0.375 |
| Hydrocarbons | ||||||||||||
| 114 | Pentadecane | 14:54.0 | 00:02.7 | S, MS, LRI | 1496 | 1500 | 1.699 | 0.251 ± 0.055 | 0.195 ± 0.066 | 0.222 ± 0.104 | 0.205 ± 0.034 | 0.140 ± 0.045 |
| 115 | 2,3,3-Trimethyl-cis-4-nonene | 10:42.0 | 00:01.6 | MS | 1313 | - | 1.585 | 0.078 ± 0.095 | 0.052 ± 0.034 | 0.031 ± 0.008 | 0.016 ± 0.009 | 0.026 ± 0.031 |
| 116 | Hexadecane | 17:00.0 | 00:02.7 | S, MS, LRI | 1600 | 1600 | 1.332 | 0.170 ± 0.025 | 0.140 ± 0.051 | 0.162 ± 0.082 | 0.134 ± 0.038 | 0.100 ± 0.031 |
| 117 | cis,trans-1,3,5-Octatriene | 08:08.4 | 00:00.7 | MS | 1196 | - | 1.087 | 0.376 ± 0.146 | 0.364 ± 0.125 | 0.315 ± 0.140 | 0.245 ± 0.137 | 0.329 ± 0.029 |
| 118 | 2,6,8-Trimethyl-trans-4-nonene | 11:13.5 | 00:02.5 | MS | 1338 | - | 0.464 | 0.048 ± 0.083 | 0.029 ± 0.052 | 0.019 ± 0.039 | 0.032 ± 0.043 | 0.002 ± 0.002 |
| Aldehydes | ||||||||||||
| 119 | Decanal | 14:54.0 | 00:01.5 | S, MS, LRI | 1496 | 1497 | 3.149 | 0.068 ± 0.041 a | 0.068 ± 0.035 a | 0.051 ± 0.040 ab | 0.014 ±0.011 b | 0.060 ± 0.008 ab |
| 120 | trans-2-Decenal | 17:49.0 | 00:01.4 | MS, LRI | 1651 | 1647 | 2.553 | 0.116 ± 0.030 | 0.131 ± 0.043 | 0.109 ± 0.030 | 0.071 ± 0.034 | 0.094 ± 0.065 |
| 121 | trans-2-Octenal | 13:25.3 | 00:01.3 | S, MS, LRI | 1435 | 1432 | 2.307 | 0.249 ± 0.301 | 0.017 ± 0.023 | 0.120 ± 0.038 | 0.105 ± 0.092 | 0.021 ± 0.020 |
| 122 | Undecanal | 17:07.0 | 00:01.5 | S, MS, LRI | 1607 | 1606 | 1.967 | 0.061 ± 0.020 | 0.053 ± 0.016 | 0.040 ± 0.010 | 0.041 ± 0.010 | 0.051 ± 0.031 |
| 123 | Dodecanal | 19:06.0 | 00:01.6 | MS, LRI | 1727 | 1722 | 1.471 | 0.066 ± 0.015 | 0.062 ± 0.019 | 0.050 ± 0.014 | 0.056 ± 0.022 | 0.043 ± 0.011 |
| 124 | 3,3-Dimethyl-2-oxobutanal | 11:24.8 | 00:01.9 | MS | 1347 | - | 0.279 | 0.108 ± 0.091 | 0.158 ± 0.178 | 0.106 ± 0.104 | 0.226 ± 0.522 | 0.078 ± 0.082 |
| 125 | Nonanal | 12:40.6 | 00:01.5 | S, MS, LRI | 1405 | 1404 | 0.206 | 10.699 ± 7.689 | 12.183 ± 7.410 | 11.470 ± 8.481 | 8.722 ± 6.668 | 10.800 ± 6.868 |
| Ketones | ||||||||||||
| 126 | 1,4,7,10,13-Pentaoxacyclononadecane-14,19-dione | 27:35.0 | 00:01.3 | MS | >2100 | - | 9.721 | 0.027 ± 0.038 b | 0.013 ± 0.014 b | 0.005 ± 0.003 b | 0.022 ± 0.020 b | 0.107 ± 0.038 a |
| 127 | α-Isophorone | 16:46.0 | 00:01.3 | S, MS, LRI | 1588 | 1593 | 7.380 | 0.116 ± 0.022 a | 0.101 ± 0.032 a | 0.047 ± 0.024 b | 0.093 ± 0.021 a | 0.093 ± 0.029 a |
| 128 | Cyclohexylideneacetone | 17:35.0 | 00:01.8 | MS | 1637 | - | 6.967 | 0.097 ± 0.097 c | 0.671 ± 0.428 b | 0.424 ± 0.259 bc | 0.546 ± 0.254 b | 1.194 ± 0.691 a |
| 129 | Acetophenone | 17:56.0 | 00:01.1 | S, MS, LRI | 1659 | 1660 | 6.036 | 0.297 ± 0.048 cd | 0.557 ± 0.116 a | 0.415 ± 0.107 bc | 0.271 ± 0.078 d | 0.487 ± 0.348 ab |
| 130 | 2-Undecanone | 16:53.4 | 00:01.5 | MS, LRI | 1594 | 1598 | 4.027 | 0.755 ± 0.471 a | 0.320 ± 0.097 b | 0.294 ± 0.104 b | 0.347 ± 0.246 b | 0.215 ± 0.162 b |
| 131 | 4,4-(Ethylenedioxy)-2-pentanone | 19:20.0 | 00:01.1 | MS | 1739 | - | 3.787 | 0.166 ± 0.053 ab | 0.245 ± 0.111 a | 0.145 ± 0.089 b | 0.102 ± 0.020 b | 0.102 ± 0.067 b |
| 132 | Unsaturated diketone (n.i.; m/z 43, 99, 71) |
14:26.0 | 00:01.1 | MS | 1477 | - | 3.213 | 0.137 ± 0.151 b | 0.361 ± 0.123 a | 0.269 ± 0.095 ab | 0.170 ± 0.174 b | 0.150 ± 0.114 b |
| 133 | 3-Undecanone | 16:20.3 | 00:01.6 | MS, LRI | 1567 | 1571 | 3.176 | 0.455 ± 0.440 a | 0.075 ± 0.051 b | 0.081 ± 0.040 b | 0.123 ± 0.265 b | 0.025 ± 0.021 b |
| 134 | 3-Tridecanone | 20:16.0 | 00:01.7 | MS, LRI | 1787 | 1755 | 3.120 | 0.036 ± 0.023 a | 0.010 ± 0.008 b | 0.008 ± 0.009 b | 0.018 ± 0.026 ab | 0.006 ± 0.005 b |
| 135 | 1b,5,5,6a-Tetramethyl-octahydro-1-oxa-cyclopropa[a]inden-6-one | 18:31.0 | 00:02.1 | MS | 1695 | - | 2.570 | 0.016 ± 0.019 | 0.042 ± 0.038 | 0.047 ± 0.054 | 0.088 ± 0.068 | 0.123 ± 0.138 |
| 136 | 3-(Acetoxy)-4-methyl-2-pentanone | 14:07.3 | 00:01.3 | MS | 1464 | - | 2.528 | 0.012 ± 0.021 | 0.055 ± 0.055 | 0.073 ± 0.057 | 0.025 ± 0.025 | 0.031 ± 0.027 |
| 137 | trans-5-Methyl-2-(1-methylethyl)-cyclohexanone | 14:08.1 | 00:01.6 | MS, LRI | 1464 | 1473 | 2.282 | 0.041 ± 0.045 | 0.312 ± 0.657 | 0.077 ± 0.050 | 0.058 ± 0.030 | 1.167 ± 1.900 |
| 138 | 4-(1,1-Dimethylethyl)-cyclohexanone | 17:35.0 | 00:01.5 | MS, LRI | 1637 | 1645 | 2.032 | 0.057 ± 0.095 | 0.124 ± 0.122 | 0.025 ± 0.033 | 0.011 ± 0.011 | 0.092 ± 0.125 |
| 139 | 1-Phenyl-1-propanone | 19:20.2 | 00:01.2 | MS, LRI | 1739 | 1744 | 1.745 | 0.019 ± 0.018 | 0.030 ± 0.013 | 0.015 ± 0.007 | 0.015 ± 0.004 | 0.021 ± 0.005 |
| 140 | 2-Nonanone | 12:34.0 | 00:01.4 | S, MS, LRI | 1401 | 1402 | 1.689 | 10.162 ± 9.346 | 4.449 ± 3.737 | 6.475 ± 7.123 | 3.195 ± 1.103 | 2.225 ± 2.037 |
| 141 | 2H-Pyran-2,6(3H)-dione | 24:10.5 | 00:00.8 | MS | 1995 | - | 1.599 | 0.485 ± 0.190 | 0.661 ± 0.326 | 0.564 ± 0.226 | 0.397 ± 0.138 | 0.394 ± 0.069 |
| 142 | 2-Heptanone | 07:46.5 | 00:05.2 | S, MS, LRI | 1180 | 1180 | 1.445 | 0.692 ± 0.406 | 0.605 ± 0.416 | 1.117 ± 1.197 | 0.301 ± 0.265 | 0.502 ± 0.401 |
| 143 | 2,2-Dimethyl-1,3-dioxane-4,6-dione | 18:17.0 | 00:01.1 | MS | 1681 | - | 1.167 | 0.032 ± 0.014 | 0.022 ± 0.021 | 0.037 ± 0.010 | 0.035 ± 0.006 | 0.026 ± 0.023 |
| 144 | 2-Decanone | 14:47.0 | 00:01.5 | MS, LRI | 1491 | 1491 | 0.990 | 0.502 ± 0.298 | 0.457 ± 0.128 | 0.377 ± 0.175 | 0.432 ± 0.159 | 0.255 ± 0.107 |
| 145 | Acetoin | 10:07.9 | 00:00.8 | S, MS, LRI | 1287 | 1287 | 0.732 | 0.054 ± 0.017 | 0.071 ± 0.048 | 0.061 ± 0.026 | 0.093 ± 0.083 | 0.058 ± 0.024 |
| 146 | 2,6-Di(tert-butyl)-4-hydroxy-4-methyl-2,5-cyclohexadien-1-one | 25:45.0 | 00:01.1 | MS, LRI | 2077 | 2094 | 0.694 | 0.009 ± 0.003 | 0.010 ± 0.006 | 0.010 ± 0.007 | 0.014 ± 0.011 | 0.013 ± 0.008 |
| 147 | 2-Cyclohexene-1,4-dione | 19:33.7 | 00:01.0 | MS | 1751 | - | 0.669 | 0.006 ± 0.010 | 0.027 ± 0.031 | 0.093 ± 0.214 | 0.031 ± 0.060 | 0.055 ± 0.078 |
| 148 | 3,4-Dihydroxy-cyclobutene-1,2-dione | 18:10.0 | 00:01.1 | MS | 1673 | - | 0.409 | 0.089 ± 0.031 | 0.075 ± 0.037 | 0.060 ± 0.053 | 0.078 ± 0.059 | 0.073 ± 0.022 |
| 149 | 5-Methyl-5-hepten-2-one | 11:24.0 | 00:01.3 | MS, LRI | 1346 | 1343 | 0.215 | 0.147 ± 0.160 | 0.113 ± 0.085 | 0.125 ± 0.023 | 0.116 ± 0.112 | 0.170 ± 0.130 |
| Alcohols | ||||||||||||
| 150 | 4-Methyl-1-heptanol | 12:42.1 | 00:01.6 | MS, LRI | 1406 | 1409 | 23.056 | 0.313 ± 0.161 b | 0.115 ± 0.060 c | 0.056 ± 0.047 c | 0.033 ± 0.031 c | 0.638 ± 0.209 a |
| 151 | cis-3-Hexen-1-ol | 12:20.0 | 00:00.9 | S, MS, LRI | 1390 | 1386 | 15.611 | 7.191 ± 2.621 c | 15.988 ± 3.409 a | 11.216 ± 4.880 b | 3.383 ± 0.607 d | 5.727 ± 3.198 cd |
| 152 | 2-Heptanol | 10:56.0 | 00:01.0 | S, MS, LRI | 1324 | 1320 | 7.290 | 0.943 ± 0.327 bc | 1.984 ± 0.923 a | 1.044 ± 0.321 bc | 0.601 ± 0.251 c | 1.571 ± 0.480 ab |
| 153 | 2-Penten-1-ol | 10:56.2 | 00:00.8 | MS, LRI | 1324 | 1321 | 5.588 | 0.044 ± 0.021 a | 0.045 ± 0.014 a | 0.044 ± 0.017 a | 0.012 ± 0.002 b | 0.040 ± 0.012 a |
| 154 | 3-Octanol | 12:36.4 | 00:01.1 | MS, LRI | 1402 | 1406 | 5.108 | 0.082 ± 0.068 b | 0.185 ± 0.070 a | 0.062 ± 0.053 b | 0.072 ± 0.050 b | 0.054 ± 0.054 b |
| 155 | 1-Undecanol | 21:49.4 | 00:01.1 | MS, LRI | 1871 | 1883 | 5.052 | 0.004 ± 0.006 b | 0.023 ± 0.015 a | 0.007 ± 0.012 b | 0.022 ± 0.010 a | 0.022 ± 0.007 a |
| 156 | Alcohol (n.i.; m/z 69, 41, 84) | 15:02.3 | 00:01.0 | MS | 1501 | - | 4.278 | 0.491 ± 0.778 bc | 0.848 ± 0.393 ab | 1.178 ± 0.637 a | 0.192 ± 0.081 c | 0.079 ± 0.036 c |
| 157 | 1-Octen-3-ol | 13:51.0 | 00:01.0 | S, MS, LRI | 1453 | 1452 | 3.832 | 3.494 ± 2.869 b | 5.797 ± 1.668 a | 2.467 ± 0.654 b | 2.597 ± 1.254 b | 3.015 ± 1.251 b |
| 158 | 2-Decanol | 17:20.3 | 00:01.1 | MS, LRI | 1621 | 1621 | 3.058 | 0.023 ± 0.011 b | 0.047 ± 0.025 b | 0.021 ± 0.015 b | 0.024 ± 0.013 b | 0.212 ± 0.318 a |
| 159 | 2,3-Butanediol II | 16:25.2 | 00:00.8 | S, MS, LRI | 1571 | 1567 | 2.708 | 1.964 ± 0.450 | 2.601 ± 0.580 | 2.586 ± 1.276 | 1.421 ± 0.780 | 2.007 ± 0.180 |
| 160 | 4-Hepten-1-ol | 14:54.5 | 00:00.9 | MS, LRI | 1496 | 1502 | 2.635 | 0.105 ± 0.065 | 0.093 ± 0.090 | 0.125 ± 0.060 | 0.044 ± 0.025 | 0.172 ± 0.057 |
| 161 | 6-Methyl-5-hepten-2-ol | 14:05.8 | 00:01.0 | S, MS, LRI | 1463 | 1466 | 2.465 | 0.034 ± 0.006 | 0.050 ± 0.015 | 0.042 ± 0.013 | 0.037 ± 0.011 | 0.030 ± 0.012 |
| 162 | 3-Methyl-1-pentanol | 11:03.7 | 00:00.9 | S, MS, LRI | 1330 | 1332 | 2.387 | 5.790 ± 1.421 | 5.799 ± 1.504 | 6.404 ± 2.937 | 3.575 ± 1.820 | 4.453 ± 0.203 |
| 163 | 2,3-Butanediol I | 15:36.0 | 00:02.1 | S, MS, LRI | 1530 | 1542 | 2.291 | 3.399 ± 1.779 | 4.339 ± 1.812 | 3.931 ± 2.279 | 1.744 ± 1.027 | 3.597 ± 0.875 |
| 164 | Alcohol (n.i.; m/z 45, 55, 43) | 14:17.6 | 00:01.0 | MS | 1471 | - | 2.157 | 0.137 ± 0.120 | 0.012 ± 0.022 | 0.096 ± 0.114 | 0.093 ± 0.042 | 0.054 ± 0.043 |
| 165 | 1-Decanol | 20:02.0 | 00:01.1 | S, MS, LRI | 1775 | 1778 | 2.086 | 0.710 ± 0.219 | 0.625 ± 0.100 | 0.597 ± 0.138 | 0.812 ± 0.097 | 0.642 ± 0.209 |
| 166 | 3,5-Dimethyl-4-heptanol | 19:35.2 | 00:00.8 | MS | 1752 | - | 2.033 | 0.043 ± 0.031 | 0.104 ± 0.063 | 0.098 ± 0.058 | 0.053 ± 0.056 | 0.068 ± 0.002 |
| 167 | 2-Ethylhexanol | 14:40.0 | 00:01.0 | MS, LRI | 1486 | 1484 | 1.756 | 4.569 ± 0.735 | 4.664 ± 0.537 | 4.826 ± 0.481 | 4.169 ± 0.465 | 4.122 ± 0.201 |
| 168 | 8-Methyl-1,8-nonanediol | 10:56.2 | 00:01.1 | MS | 1324 | - | 1.454 | 0.123 ± 0.079 | 0.199 ± 0.084 | 0.223 ± 0.122 | 0.206 ± 0.084 | 0.133 ± 0.116 |
| 169 | 3,4-Nonadienol | 19:48.0 | 00:01.0 | MS, LRI | 1763 | 1754 | 1.445 | 0.017 ± 0.025 | 0.016 ± 0.013 | 0.007 ± 0.005 | 0.003 ± 0.002 | 0.005 ± 0.001 |
| 170 | 1-Pentanol | 08:53.5 | 00:00.5 | S, MS, LRI | 1231 | 1242 | 1.272 | 0.082 ± 0.091 | 0.135 ± 0.100 | 0.112 ± 0.076 | 0.058 ± 0.060 | 0.039 ± 0.014 |
| 171 | 3-Ethyl-4-octanol | 18:24.9 | 00:01.3 | MS | 1689 | - | 1.225 | 0.364 ± 0.208 | 0.304 ± 0.189 | 0.479 ± 0.198 | 0.471 ± 0.108 | 0.331 ± 0.229 |
| 172 | 2-Octen-1-ol | 17:14.0 | 00:01.0 | S, MS, LRI | 1615 | 1622 | 1.115 | 0.151 ± 0.206 | 0.114 ± 0.060 | 0.077 ± 0.033 | 0.041 ± 0.015 | 0.057 ± 0.020 |
| 173 | 2-Undecanol | 19:13.0 | 00:01.2 | MS, LRI | 1733 | 1738 | 0.841 | 0.173 ± 0.218 | 0.330 ± 0.184 | 0.226 ± 0.137 | 0.241 ± 0.094 | 0.278 ± 0.205 |
| 174 | 4-Methyl-1-pentanol | 10:49.0 | 00:00.9 | MS, LRI | 1319 | 1319 | 0.819 | 2.297 ± 1.083 | 2.626 ± 0.800 | 3.318 ± 2.010 | 2.130 ± 2.101 | 1.782 ± 0.130 |
| 175 | 4-Ethyl-3-octanol | 15:08.0 | 00:01.2 | MS | 1506 | - | 0.721 | 0.400 ± 0.059 | 0.374 ± 0.067 | 0.430 ± 0.232 | 0.389 ± 0.202 | 0.245 ± 0.213 |
| 176 | 2-Nonanol | 15:15.0 | 00:01.1 | S, MS, LRI | 1512 | 1518 | 0.715 | 0.512 ± 0.187 | 0.581 ± 0.344 | 0.429 ± 0.127 | 0.398 ± 0.169 | 0.468 ± 0.272 |
| 177 | 1-Nonanol | 18:03.3 | 00:01.0 | S, MS, LRI | 1666 | 1661 | 0.580 | 1.420 ± 1.635 | 0.776 ± 0.538 | 2.515 ± 3.679 | 1.557 ± 2.268 | 1.018 ± 1.218 |
| 178 | 1-Heptanol | 13:58.2 | 00:00.9 | S, MS, LRI | 1458 | 1457 | 0.560 | 1.238 ± 0.721 | 1.394 ± 0.657 | 1.120 ± 0.904 | 0.875 ± 0.381 | 1.183 ± 0.341 |
| 179 | 2-Methyl-1-pentanol | 10:28.5 | 00:00.9 | S, MS, LRI | 1303 | 1297 | 0.229 | 0.262 ± 0.326 | 0.200 ± 0.248 | 0.323 ± 0.434 | 0.312 ± 0.145 | 0.199 ± 0.124 |
| 180 | trans-4-tert-Butylcyclohexanol | 19:43.3 | 00:01.1 | MS, LRI | 1759 | 1730 | 0.189 | 0.136 ± 0.326 | 0.149 ± 0.283 | 0.267 ± 0.447 | 0.204 ± 0.181 | 0.216 ± 0.332 |
| 181 | 2-Octanol (internal standard) | 13:09.0 | 00:01.0 | S, MS, LRI | 1424 | 1418 | 40.000 ± 0.000 | 40.000 ± 0.000 | 40.000 ± 0.000 | 40.000 ± 0.000 | 40.000 ± 0.000 | |
| Acids | ||||||||||||
| 182 | Propionic acid | 15:43.0 | 00:00.7 | S, MS, LRI | 1536 | 1540 | 4.365 | 1.294 ± 0.324 ab | 1.631 ± 0.472 a | 1.159 ± 0.294 b | 0.946 ± 0.158 b | 0.991 ± 0.348 b |
| 183 | Acid (n.i.; m/z 74, 45, 73) | 14:33.0 | 00:01.1 | MS, LRI | 1481 | 1491 | 4.041 | 0.018 ± 0.015 b | 0.038 ± 0.020 a | 0.016 ± 0.013 b | 0.009 ± 0.006 b | 0.013 ± 0.008 b |
| 184 | trans-2-Hexenoic acid | 23:39.0 | 00:00.8 | MS, LRI | 1967 | 1967 | 3.651 | 0.081 ± 0.047 b | 0.205 ± 0.094 a | 0.159 ± 0.107 ab | 0.083 ± 0.045 b | 0.266 ± 0.213 a |
| 185 | Nonanoic acid | 26:51.5 | 00:00.8 | S, MS, LRI | >2100 | 2119 | 3.641 | 0.096 ± 0.049 b | 0.169 ± 0.095 b | 0.094 ± 0.043 b | 0.166 ± 0.156 b | 0.313 ± 0.078 a |
| 186 | trans-3-Hexenoic acid | 22:49.8 | 00:00.8 | MS, LRI | 1924 | 1929 | 3.190 | 0.031 ± 0.033 a | 0.006 ± 0.005 b | 0.007 ± 0.003 b | 0.005 ± 0.003 b | 0.011 ± 0.005 ab |
| 187 | Formic acid | 15:10.4 | 00:00.7 | MS, LRI | 1508 | 1501 | 2.526 | 1.442 ± 0.465 | 2.092 ± 0.849 | 1.523 ± 0.393 | 1.176 ± 0.507 | 1.306 ± 0.515 |
| 188 | 3,5,5-Trimethylhexanoic acid | 23:46.0 | 00:00.8 | MS | 1973 | - | 2.194 | 0.330 ± 0.053 | 0.347 ± 0.115 | 0.370 ± 0.113 | 0.437 ± 0.094 | 0.479 ± 0.101 |
| 189 | 2-Propenoic acid | 17:42.7 | 00:00.7 | MS | 1645 | - | 2.118 | 0.245 ± 0.073 | 0.262 ± 0.101 | 0.266 ± 0.042 | 0.214 ± 0.039 | 0.146 ± 0.055 |
| 190 | Heptanoic acid | 23:22.2 | 00:00.8 | S, MS, LRI | 1953 | 1955 | 1.423 | 0.071 ± 0.022 | 0.075 ± 0.044 | 0.083 ± 0.076 | 0.060 ± 0.021 | 0.152 ± 0.139 |
| 191 | 2-Decenoic acid | 15:43.7 | 00:00.8 | MS, LRI | 1536 | 1540 | 1.289 | 0.021 ± 0.022 | 0.012 ± 0.024 | 0.005 ± 0.010 | 0.025 ± 0.011 | 0.022 ± 0.025 |
| 192 | Pentanoic acid | 19:34.0 | 00:00.8 | S, MS, LRI | 1751 | 1751 | 1.006 | 0.408 ± 0.074 | 0.490 ± 0.147 | 0.395 ± 0.074 | 0.394 ± 0.063 | 0.513 ± 0.338 |
| 193 | Isobutyric acid | 16:18.0 | 00:00.7 | S, MS, LRI | 1565 | 1555 | 0.832 | 3.347 ± 0.988 | 4.725 ± 1.518 | 4.212 ± 2.107 | 3.795 ± 1.757 | 3.200 ± 2.305 |
| 194 | trans,trans-2,4-Hexadienoic acid | 26:51.6 | 00:00.8 | MS, LRI | >2100 | 2150 | 0.753 | 0.188 ± 0.145 | 0.053 ± 0.066 | 18.360 ± 48.172 | 4.350 ± 10.563 | 2.236 ± 3.744 |
| 195 | Isovaleric acid | 18:18.8 | 00:00.7 | S, MS, LRI | 1683 | 1680 | 0.745 | 5.833 ± 1.482 | 3.926 ± 3.916 | 5.923 ± 2.842 | 6.130 ± 2.208 | 5.222 ± 2.990 |
| 196 | 2-Ethylhexanoic acid | 23:18.0 | 00:00.8 | MS, LRI | 1949 | 1960 | 0.568 | 0.602 ± 1.256 | 0.232 ± 0.224 | 0.312 ± 0.271 | 0.144 ± 0.061 | 0.151 ± 0.019 |
| 197 | Butyric acid | 17:28.0 | 00:00.7 | S, MS, LRI | 1629 | 1626 | 0.546 | 18.241 ± 2.608 | 19.305 ± 5.118 | 17.622 ± 2.372 | 16.638 ± 0.905 | 18.144 ± 6.443 |
| Esters | ||||||||||||
| 198 | Methyl octanoate | 12:34.0 | 00:01.5 | MS, LRI | 1401 | 1404 | 12.568 | 51.242 ± 14.675 a | 14.588 ± 8.497 b | 25.349 ± 13.750 b | 21.407 ± 4.375 b | 12.261 ± 13.962 b |
| 199 | cis-3-Hexen-1-yl acetate | 10:56.0 | 00:01.3 | MS, LRI | 1324 | 1300 | 12.068 | 20.041 ± 8.968 b | 39.339 ± 11.050 a | 22.891 ± 14.489 b | 6.372 ± 2.659 c | 4.576 ± 3.341 c |
| 200 | Methyl hexanoate | 07:51.1 | 00:05.4 | S, MS, LRI | 1183 | 1188 | 10.455 | 6.240 ± 2.416 a | 2.417 ± 1.319 b | 3.001 ± 1.098 b | 2.216 ± 0.469 b | 1.222 ± 1.089 b |
| 201 | Butyl hexanoate | 13:05.2 | 00:01.7 | S, MS, LRI | 1422 | 1428 | 10.423 | 0.059 ± 0.026 a | 0.015 ± 0.014 b | 0.017 ± 0.016 b | 0.015 ± 0.008 b | 0.008 ± 0.007 b |
| 202 | Isoamyl hexanoate | 14:05.0 | 00:01.8 | S, MS, LRI | 1462 | 1458 | 9.888 | 7.130 ± 1.573 a | 1.804 ± 1.100 c | 3.941 ± 3.105 b | 3.323 ± 0.756 bc | 1.537 ± 1.597 c |
| 203 | Ethyl 3-nonenoate | 16:44.2 | 00:01.6 | MS | 1587 | - | 7.481 | 0.067 ± 0.040 a | 0.021 ± 0.020 b | 0.011 ± 0.005 b | 0.017 ± 0.015 b | 0.006 ± 0.007 b |
| 204 | Hexanodibutyrin | 12:20.0 | 00:01.7 | MS | 1390 | - | 7.472 | 0.444 ± 0.181 a | 0.571 ± 0.245 a | 0.180 ± 0.173 b | 0.179 ± 0.103 b | 0.705 ± 0.336 a |
| 205 | Methyl decanoate | 16:53.0 | 00:01.6 | MS, LRI | 1594 | 1593 | 7.131 | 1.375 ± 0.608 a | 0.552 ± 0.226 b | 0.741 ± 0.248 b | 0.599 ± 0.132 b | 0.426 ± 0.345 b |
| 206 | Phenethyl formate | 20:29.8 | 00:01.1 | MS, LRI | 1799 | 1806 | 6.718 | 0.088 ± 0.035 c | 0.211 ± 0.070 a | 0.156 ± 0.046 b | 0.119 ± 0.043 bc | 0.112 ± 0.032 bc |
| 207 | Ethyl trans-4-octenoate | 15:15.0 | 00:01.5 | MS | 1512 | - | 6.256 | 0.031 ± 0.003 a | 0.017 ± 0.009 b | 0.023 ± 0.006 b | 0.016 ± 0.007 b | 0.017 ± 0.006 b |
| 208 | Ethyl methyl succinate | 17:36.4 | 00:01.1 | MS, LRI | 1638 | 1631 | 6.026 | 0.347 ± 0.156 a | 0.306 ± 0.126 a | 0.242 ± 0.078 a | 0.079 ± 0.067 b | 0.254 ± 0.070 a |
| 209 | Octyl formate | 16:04.2 | 00:01.0 | MS, LRI | 1553 | 1560 | 5.909 | 6.604 ± 0.947 b | 6.267 ± 1.160 b | 6.531 ± 1.465 b | 9.177 ± 1.345 a | 6.050 ± 2.280 b |
| 210 | trans-3-Hexen-1-yl acetate | 10:42.0 | 00:01.3 | MS, LRI | 1313 | 1316 | 5.675 | 20.322 ± 7.276 a | 10.073 ± 8.187 b | 5.922 ± 3.176 b | 10.530 ± 4.255 b | 7.388 ± 8.444 b |
| 211 | Ethyl hexadecanoate | 30:13.7 | 00:01.5 | MS, LRI | >2100 | 2261 | 5.586 | 0.560 ± 0.414 a | 0.051 ± 0.082 b | 0.119 ± 0.222 b | 0.091 ± 0.149 b | 0.052 ± 0.064 b |
| 212 | Ethyl 2-hydroxy-4-methylpentanoate | 15:49.3 | 00:01.0 | MS | 1541 | - | 5.543 | 0.380 ± 0.161 c | 0.829 ± 0.571 bc | 1.875 ± 0.999 a | 1.625 ± 1.112 ab | 0.266 ± 0.231 c |
| 213 | 2-Phenylethyl isobutyrate | 22:45.7 | 00:01.1 | MS, LRI | 1921 | 1916 | 5.419 | 3.788 ± 1.880 a | 1.824 ± 1.708 b | 1.421 ± 1.243 b | 0.816 ± 0.397 b | 0.769 ± 0.245 b |
| 214 | Propyl hexanoate | 10:56.2 | 00:01.7 | MS, LRI | 1324 | 1319 | 4.982 | 0.760 ± 0.330 a | 0.339 ± 0.286 b | 0.286 ± 0.227 b | 0.379 ± 0.143 b | 0.170 ± 0.180 b |
| 215 | Diethyl glutarate | 20:23.0 | 00:01.2 | MS, LRI | 1793 | 1780 | 4.706 | 0.028 ± 0.012 c | 0.105 ± 0.061 a | 0.064 ± 0.043 bc | 0.059 ± 0.013 bc | 0.092 ± 0.020 ab |
| 216 | 3-Ethoxypropyl acetate | 11:52.0 | 00:01.2 | MS | 1368 | - | 4.423 | 0.754 ± 0.514 b | 2.176 ± 1.442 a | 0.638 ± 0.941 b | 0.442 ± 0.365 b | 0.454 ± 0.707 b |
| 217 | Isoamyl octanoate | 18:06.1 | 00:01.9 | MS, LRI | 1669 | 1657 | 4.033 | 4.133 ± 0.747 a | 2.340 ± 0.822 b | 3.155 ± 1.362 ab | 3.789 ± 0.950 a | 2.125 ± 1.656 b |
| 218 | Ethyl heptanoate | 11:17.0 | 00:01.6 | MS, LRI | 1341 | 1342 | 3.762 | 3.007 ± 1.131 a | 1.591 ± 1.355 b | 1.701 ± 0.709 b | 1.498 ± 0.355 b | 0.938 ± 1.126 b |
| 219 | Ethyl pyruvate | 09:53.4 | 00:01.0 | MS, LRI | 1276 | 1276 | 3.717 | 1.711 ± 0.693 b | 3.025 ± 0.908 a | 1.931 ± 1.095 b | 1.496 ± 0.650 b | 1.685 ± 0.421 b |
| 220 | Isoamyl decanoate | 21:58.0 | 00:01.9 | MS, LRI | 1879 | 1871 | 3.685 | 0.039 ± 0.093 b | 0.191 ± 0.114 ab | 0.180 ± 0.153 ab | 0.239 ± 0.042 ab | 0.178 ± 0.115 ab |
| 221 | Propyl octanoate | 15:22.0 | 00:01.8 | MS, LRI | 1518 | 1504 | 3.637 | 0.893 ± 0.277 a | 0.428 ± 0.431 b | 0.384 ± 0.269 b | 0.577 ± 0.297 ab | 0.270 ± 0.261 b |
| 222 | Ethyl 4-pyrazolecarboxylate | 14:33.0 | 00:01.1 | MS | 1481 | - | 3.291 | 0.016 ± 0.002 b | 0.026 ± 0.011 a | 0.014 ± 0.010 b | 0.012 ± 0.008 b | 0.010 ± 0.009 b |
| 223 | Methyl 2-methyllactate | 16:39.0 | 00:01.2 | MS | 1582 | - | 3.049 | 0.116 ± 0.015 a | 0.088 ± 0.013 bc | 0.102 ± 0.017 ab | 0.109 ± 0.024 ab | 0.068 ± 0.059 c |
| 224 | 3-Hydroxy-2,4,4-trimethylpentyl isobutyrate | 21:54.7 | 00:01.2 | MS | 1876 | - | 3.046 | 0.089 ± 0.019 b | 0.191 ± 0.051 a | 0.124 ± 0.108 b | 0.104 ± 0.035 b | 0.112 ± 0.044 b |
| 225 | Isobutyl hexanoate | 11:45.0 | 00:01.8 | MS, LRI | 1362 | 1357 | 2.919 | 1.039 ± 0.567 a | 0.392 ± 0.297 b | 0.767 ± 0.580 ab | 0.595 ± 0.202 ab | 0.259 ± 0.272 b |
| 226 | Hydroxyl acid ester (n.i.; m/z 143, 115, 75) |
17:49.0 | 00:01.3 | MS | 1651 | - | 2.680 | 0.005 ± 0.005 | 0.012 ± 0.005 | 0.009 ± 0.006 | 0.007 ± 0.004 | 0.014 ± 0.005 |
| 227 | Ethyl 4-hydroxybutyrate | 20:47.7 | 00:00.9 | MS, LRI | 1815 | 1819 | 2.518 | 0.955 ± 0.717 | 1.134 ± 0.677 | 1.141 ± 0.695 | 0.315 ± 0.171 | 0.536 ± 0.197 |
| 228 | Octyl acetate | 14:24.4 | 00:01.5 | MS, LRI | 1475 | 1475 | 2.454 | 0.408 ± 0.857 | 1.601 ± 1.141 | 1.423 ± 1.416 | 3.153 ± 1.639 | 6.579 ± 10.545 |
| 229 | 3-Methyl-3-buten-1-yl acetate | 08:07.8 | 00:01.2 | MS, LRI | 1196 | 1190 | 2.448 | 0.072 ± 0.037 | 0.066 ± 0.036 | 0.032 ± 0.028 | 0.049 ± 0.021 | 0.025 ± 0.026 |
| 230 | Ethyl 2-octenoate | 16:04.0 | 00:01.5 | MS, LRI | 1553 | 1557 | 2.386 | 0.031 ± 0.005 | 0.027 ± 0.009 | 0.024 ± 0.008 | 0.019 ± 0.003 | 0.027 ± 0.015 |
| 231 | Ethyl undecanoate | 18:45.2 | 00:01.0 | MS, LRI | 1709 | 1725 | 2.352 | 0.539 ± 0.749 | 0.198 ± 0.111 | 0.319 ± 0.391 | 1.006 ± 0.777 | 0.166 ± 0.169 |
| 232 | Pentyl acetate | 07:36.1 | 00:01.3 | S, MS, LRI | 1172 | 1161 | 2.334 | 0.123 ± 0.199 | 0.088 ± 0.204 | 0.037 ± 0.056 | 0.282 ± 0.174 | 0.053 ± 0.057 |
| 233 | Isobutyl octanoate | 15:57.7 | 00:01.9 | MS, LRI | 1548 | 1551 | 2.226 | 0.217 ± 0.071 | 0.119 ± 0.083 | 0.185 ± 0.114 | 0.213 ± 0.109 | 0.074 ± 0.069 |
| 234 | Ethyl 4-hexenoate | 10:21.3 | 00:01.4 | MS, LRI | 1297 | 1292 | 2.211 | 7.705 ± 10.236 | 2.044 ± 1.364 | 1.287 ± 0.650 | 0.784 ± 0.458 | 1.363 ± 1.374 |
| 235 | Ethyl trans-2-butenoate | 07:19.0 | 00:05.0 | MS, LRI | 1159 | 1161 | 2.127 | 8.551 ± 4.382 | 5.204 ± 3.185 | 7.463 ± 2.841 | 4.376 ± 2.710 | 4.293 ± 2.995 |
| 236 | trans,trans-2,4-Octadien-1-yl acetate | 16:18.0 | 00:01.4 | MS | 1565 | - | 1.956 | 0.017 ± 0.017 | 0.024 ± 0.018 | 0.006 ± 0.010 | 0.007 ± 0.009 | 0.017 ± 0.017 |
| 237 | 2-Phenylethyl octanoate | 32:17.5 | 00:01.3 | MS, LRI | >2100 | 2373 | 1.762 | 0.032 ± 0.036 | 0.006 ± 0.005 | 0.009 ± 0.004 | 0.024 ± 0.030 | 0.004 ± 0.004 |
| 238 | Isoamyl butyrate | 09:45.8 | 00:01.7 | S, MS, LRI | 1270 | 1266 | 1.756 | 2.573 ± 0.919 | 2.131 ± 0.957 | 2.055 ± 0.892 | 1.912 ± 0.358 | 1.089 ± 1.161 |
| 239 | Di-isobutyl acetate | 20:02.0 | 00:01.2 | MS | 1775 | - | 1.724 | 0.206 ± 0.065 | 0.243 ± 0.211 | 0.139 ± 0.106 | 0.112 ± 0.037 | 0.094 ± 0.032 |
| 240 | 3-Methylheptyl acetate | 12:26.5 | 00:01.6 | MS | 1395 | - | 1.665 | 0.367 ± 0.347 | 0.236 ± 0.216 | 0.182 ± 0.086 | 0.112 ± 0.050 | 0.124 ± 0.077 |
| 241 | Diethyl malonate | 16:32.0 | 00:01.1 | MS, LRI | 1577 | 1574 | 1.648 | 0.174 ± 0.052 | 0.188 ± 0.079 | 0.148 ± 0.050 | 0.126 ± 0.033 | 0.197 ± 0.034 |
| 242 | Heptyl acetate | 12:13.4 | 00:01.5 | MS, LRI | 1385 | 1385 | 1.487 | 0.236 ± 0.133 | 0.249 ± 0.179 | 0.151 ± 0.091 | 0.120 ± 0.044 | 0.143 ± 0.145 |
| 243 | Ethyl hydrogen succinate | 30:16.2 | 00:00.8 | MS, LRI | >2100 | 2350 | 1.443 | 3.279 ± 0.920 | 7.386 ± 6.573 | 5.875 ± 1.785 | 4.985 ± 2.470 | 4.242 ± 1.226 |
| 244 | Methyl 2-isopropoxypropanoate | 20:30.0 | 00:01.3 | MS | 1799 | - | 1.288 | 0.030 ± 0.042 | 0.076 ± 0.036 | 0.061 ± 0.043 | 0.065 ± 0.045 | 0.053 ± 0.047 |
| 245 | Vinyl decanoate | 19:27.2 | 00:01.4 | MS | 1745 | - | 1.258 | 0.224 ± 0.357 | 0.086 ± 0.065 | 0.044 ± 0.038 | 0.038 ± 0.034 | 0.130 ± 0.062 |
| 246 | Diethyl malate | 25:29.4 | 00:00.9 | MS, LRI | 2063 | 2065 | 1.210 | 0.227 ± 0.144 | 0.462 ± 0.502 | 0.399 ± 0.220 | 0.360 ± 0.231 | 0.629 ± 0.235 |
| 247 | trans-Penten-1-yl acetate | 08:50.0 | 00:01.2 | MS | 1228 | - | 1.132 | 0.050 ± 0.049 | 0.039 ± 0.049 | 0.024 ± 0.031 | 0.011 ± 0.011 | 0.024 ± 0.023 |
| 248 | Phenylmethyl acetate | 19:27.0 | 00:01.2 | MS, LRI | 1745 | 1747 | 1.119 | 0.042 ± 0.026 | 0.211 ± 0.394 | 0.035 ± 0.018 | 0.046 ± 0.018 | 0.054 ± 0.021 |
| 249 | Ethyl 3-hydroxybutyrate | 15:15.5 | 00:00.9 | MS, LRI | 1512 | 1512 | 1.045 | 0.398 ± 0.278 | 0.407 ± 0.202 | 0.430 ± 0.195 | 0.233 ± 0.157 | 0.430 ± 0.093 |
| 250 | Isoamyl propanoate | 07:56.8 | 00:01.5 | MS, LRI | 1188 | 1188 | 1.017 | 0.638 ± 0.264 | 0.677 ± 0.436 | 0.740 ± 0.490 | 0.543 ± 0.341 | 0.250 ± 0.186 |
| 251 | Ethyl hydroxyacetate | 13:09.0 | 00:00.8 | MS, LRI | 1424 | 1436 | 0.987 | 0.032 ± 0.045 | 0.097 ± 0.079 | 0.058 ± 0.076 | 0.085 ± 0.144 | 0.163 ± 0.215 |
| 252 | Ethyl nonanoate | 15:43.0 | 00:01.7 | MS, LRI | 1536 | 1535 | 0.928 | 1.843 ± 0.503 | 0.984 ± 0.394 | 0.971 ± 0.345 | 2.321 ± 3.355 | 1.116 ± 0.807 |
| 253 | 2-(1,1-Dimethylethyl)-cyclohexen-1-yl acetate | 16:11.0 | 00:01.8 | MS | 1559 | - | 0.848 | 0.046 ± 0.026 | 0.024 ± 0.015 | 0.045 ± 0.031 | 0.040 ± 0.032 | 0.034 ± 0.004 |
| 254 | Ethyl 2-hydroxy-3-phenylpropanoate | 29:11.1 | 00:01.0 | MS, LRI | >2100 | 2273 | 0.846 | 0.001 ± 0.001 | 0.001 ± 0.001 | 0.009 ± 0.019 | 0.007 ± 0.014 | 0.001 ± 0.000 |
| 255 | Ethyl 3-methylbutylbutanedioate | 22:29.7 | 00:01.3 | MS, LRI | 1907 | 1907 | 0.835 | 2.315 ± 0.806 | 3.353 ± 2.324 | 2.743 ± 1.814 | 2.843 ± 0.908 | 1.639 ± 0.545 |
| 256 | Ethyl 9-decenoate | 18:45.0 | 00:01.6 | S, MS, LRI | 1708 | 1708 | 0.801 | 0.133 ± 0.189 | 0.177 ± 0.227 | 0.280 ± 0.347 | 0.101 ± 0.086 | 0.067 ± 0.058 |
| 257 | Ethyl 3-ethoxy-trans-2-propenoate | 15:43.0 | 00:01.2 | MS | 1536 | - | 0.785 | 1.418 ± 0.096 | 1.363 ± 0.205 | 1.480 ± 0.136 | 1.542 ± 0.317 | 1.360 ± 0.353 |
| 258 | Butyl ethyl succinate | 20:37.0 | 00:01.3 | MS, LRI | 1806 | 1820 | 0.776 | 0.245 ± 0.112 | 0.295 ± 0.195 | 0.294 ± 0.182 | 0.220 ± 0.139 | 0.136 ± 0.063 |
| 259 | Ethyl 2,4-hexadienoate I | 14:35.0 | 00:01.3 | MS, LRI | 1483 | 1501 | 0.673 | 0.033 ± 0.034 | 0.019 ± 0.011 | 0.869 ± 2.269 | 0.343 ± 0.855 | 0.097 ± 0.153 |
| 260 | Ethyl 2,4-hexadienoate II | 15:08.0 | 00:01.3 | MS, LRI | 1506 | 1501 | 0.645 | 0.290 ± 0.328 | 0.038 ± 0.016 | 6.200 ± 16.289 | 3.473 ± 8.685 | 0.353 ± 0.515 |
| 261 | Isobutyl acetate | 04:23.8 | 00:04.7 | S, MS, LRI | 1015 | 1009 | 0.531 | 0.726 ± 0.371 | 0.774 ± 0.280 | 0.728 ± 0.435 | 0.534 ± 0.175 | 0.658 ± 0.405 |
| 262 | 2-Phenylethyl isovalerate | 24:00.0 | 00:01.4 | MS, LRI | 1985 | 1988 | 0.530 | 0.015 ± 0.012 | 0.014 ± 0.014 | 0.013 ± 0.010 | 0.020 ± 0.006 | 0.011 ± 0.010 |
| 263 | Methyl 2-hydroxybutanoate | 11:45.0 | 00:01.1 | MS, LRI | 1362 | 1382 | 0.529 | 0.274 ± 0.056 | 0.217 ± 0.155 | 0.208 ± 0.156 | 0.280 ± 0.040 | 0.225 ± 0.197 |
| 264 | Isopropyl lactate | 15:36.0 | 00:01.6 | MS | 1530 | - | 0.512 | 0.032 ± 0.007 | 0.027 ± 0.004 | 0.029 ± 0.016 | 0.026 ± 0.007 | 0.023 ± 0.024 |
| 265 | Ethyl cis-4-decenoate | 18:17.4 | 00:01.6 | MS, LRI | 1681 | 1680 | 0.510 | 0.017 ± 0.019 | 0.012 ± 0.010 | 0.008 ± 0.014 | 0.015 ± 0.011 | 0.011 ± 0.009 |
| 266 | Ethyl cis-4-octenoate | 14:39.6 | 00:01.5 | MS | 1486 | - | 0.503 | 0.127 ± 0.073 | 0.094 ± 0.054 | 0.123 ± 0.053 | 0.098 ± 0.015 | 0.101 ± 0.094 |
| 267 | Ethyl 2-hexenoate | 11:31.7 | 00:01.5 | S, MS, LRI | 1352 | 1357 | 0.469 | 1.626 ± 0.984 | 1.864 ± 0.759 | 2.107 ± 1.213 | 1.408 ± 0.806 | 1.721 ± 1.542 |
| 268 | Isoamyl lactate | 16:18.0 | 00:01.0 | MS, LRI | 1565 | 1572 | 0.376 | 0.449 ± 0.304 | 0.829 ± 1.062 | 0.597 ± 0.380 | 0.703 ± 0.581 | 0.596 ± 0.341 |
| 269 | Ethyl 2-propynoate | 09:11.0 | 00:01.8 | MS | 1244 | - | 0.368 | 4.550 ± 1.065 | 4.324 ± 1.444 | 4.768 ± 1.504 | 4.322 ± 1.565 | 3.562 ± 2.475 |
| 270 | Diethyl fumarate | 17:56.0 | 00:01.2 | MS, LRI | 1659 | 1660 | 0.155 | 0.055 ± 0.027 | 0.047 ± 0.018 | 0.048 ± 0.031 | 0.050 ± 0.014 | 0.045 ± 0.014 |
| Volatile phenols | ||||||||||||
| 271 | 2-Methoxyphenol | 21:47.0 | 00:00.9 | MS, LRI | 1869 | 1869 | 5.084 | 0.023 ± 0.016 b | 0.052 ± 0.034 a | 0.019 ± 0.011 b | 0.010 ± 0.001 b | 0.011 ± 0.003 b |
| 272 | 4-Vinylguaiacol | 27:33.5 | 00:00.9 | S, MS, LRI | >2100 | 2168 | 2.970 | 0.563 ± 0.266 ab | 0.762 ± 0.426 a | 0.373 ± 0.170 b | 0.377 ± 0.330 b | 0.163 ± 0.069 b |
| 273 | Phenol | 24:14.0 | 00:00.8 | S, MS, LRI | 1998 | 1995 | 1.607 | 0.701 ± 0.049 | 0.856 ± 0.131 | 0.804 ± 0.309 | 0.601 ± 0.130 | 0.886 ± 0.541 |
| 274 | 4-Ethylguaiacol | 24:44.7 | 00:01.0 | S, MS, LRI | 2024 | 2024 | 1.514 | 0.011 ± 0.020 | 0.024 ± 0.034 | 0.004 ± 0.004 | 0.002 ± 0.005 | 0.003 ± 0.003 |
| 275 | 2,4-Bis(1,1-dimethylethyl)phenol | 29:01.0 | 00:01.0 | MS, LRI | >2100 | 2270 | 1.328 | 0.923 ± 0.417 | 0.821 ± 0.247 | 1.133 ± 0.583 | 0.667 ± 0.280 | 0.810 ± 0.189 |
| Furanoids and lactones | ||||||||||||
| 276 | 3-Methyl-2(5H)-furanone | 19:13.3 | 00:01.0 | MS, LRI | 1733 | 1726 | 7.776 | 0.029 ± 0.012 a | 0.024 ± 0.011 ab | 0.017 ± 0.009 b | 0.004 ± 0.004 c | 0.017 ± 0.006 ab |
| 277 | Acetylfuran | 15:01.2 | 00:01.0 | MS, LRI | 1501 | 1501 | 4.958 | 0.092 ± 0.054 b | 0.416 ± 0.326 a | 0.201 ± 0.104 b | 0.086 ± 0.040 b | 0.092 ± 0.031 b |
| 278 | γ-Butyrolactone | 17:28.0 | 00:01.0 | MS, LRI | 1629 | 1626 | 4.491 | 3.839 ± 1.256 a | 4.284 ± 1.527 a | 4.055 ± 0.852 a | 1.918 ± 1.271 b | 2.661 ± 0.844 ab |
| 279 | 2-(5-Methyl-5-vinyltetrahydro-2-furanyl)-2-propanol | 14:16.1 | 00:01.2 | MS | 1470 | - | 4.293 | 0.050 ± 0.089 b | 0.106 ± 0.063 b | 0.053 ± 0.063 b | 0.100 ± 0.084 b | 0.305 ± 0.233 a |
| 280 | γ-Nonalactone | 24:42.6 | 00:01.2 | S, MS, LRI | 2022 | 2018 | 3.909 | 0.037 ± 0.072 c | 0.311 ± 0.222 a | 0.152 ± 0.123 bc | 0.200 ± 0.100 ab | 0.191 ± 0.089 abc |
| 281 | Lactone (n.i.; m/z 85, 57, 100) | 23:39.0 | 00:01.1 | MS | 1967 | - | 2.450 | 0.057 ± 0.059 | 0.013 ± 0.014 | 0.039 ± 0.037 | 0.010 ± 0.006 | 0.009 ± 0.001 |
| 282 | Pantolactone | 24:42.0 | 00:00.8 | MS, LRI | 2022 | 2029 | 2.220 | 0.091 ± 0.018 | 0.184 ± 0.075 | 0.174 ± 0.101 | 0.156 ± 0.067 | 0.123 ± 0.046 |
| 283 | Furfuryl ether | 10:14.0 | 00:01.1 | MS | 1292 | - | 2.138 | 0.159 ± 0.187 | 0.242 ± 0.117 | 0.245 ± 0.100 | 0.091 ± 0.033 | 0.116 ± 0.071 |
| 284 | Furfural | 14:05.2 | 00:00.9 | S, MS, LRI | 1462 | 1460 | 2.087 | 1.122 ± 0.381 | 16.951 ± 26.840 | 2.258 ± 1.044 | 0.857 ± 0.168 | 1.010 ± 0.306 |
| 285 | Ethyl 2-furoate | 17:21.0 | 00:01.1 | MS, LRI | 1622 | 1624 | 1.823 | 4.805 ± 1.288 | 7.089 ± 2.513 | 4.929 ± 2.119 | 5.484 ± 1.375 | 4.786 ± 1.742 |
| 286 | γ-Octalactone | 22:49.8 | 00:01.1 | S, MS, LRI | 1924 | 1923 | 1.722 | 0.533 ± 0.208 | 0.907 ± 0.309 | 0.708 ± 0.325 | 0.723 ± 0.299 | 0.730 ± 0.048 |
| 287 | 2(5H)-furanone | 19:59.4 | 00:00.9 | S, MS, LRI | 1773 | 1787 | 1.266 | 0.079 ± 0.013 | 0.151 ± 0.172 | 0.078 ± 0.013 | 0.060 ± 0.010 | 0.079 ± 0.046 |
| 288 | 5-Methyl-2-furfural | 16:25.8 | 00:01.0 | S, MS, LRI | 1571 | 1570 | 1.263 | 0.014 ± 0.014 | 1.971 ± 4.278 | 0.055 ± 0.053 | 0.033 ± 0.013 | 0.027 ± 0.033 |
| 289 | Lactone (n.i.; m/z 99, 71, 87) | 23:41.8 | 00:01.2 | MS | 1970 | - | 1.206 | 0.012 ± 0.022 | 0.121 ± 0.223 | 0.022 ± 0.036 | 0.025 ± 0.022 | 0.040 ± 0.034 |
| 290 | γ-Hydroxymethyl-γ-butyrolactone | 28:33.0 | 00:00.9 | MS | >2100 | - | 0.725 | 1.594 ± 1.045 | 1.857 ± 1.170 | 2.753 ± 1.813 | 2.442 ± 1.912 | 1.735 ± 1.520 |
| 291 | 5-Ethoxydihydro-2(3H)-furanone | 19:20.0 | 00:01.0 | MS, LRI | 1739 | 1728 | 0.666 | 0.054 ± 0.024 | 0.059 ± 0.031 | 0.071 ± 0.032 | 0.046 ± 0.027 | 0.062 ± 0.039 |
| 292 | δ-Caprolactone | 20:37.4 | 00:01.1 | MS, LRI | 1806 | 1818 | 0.121 | 0.344 ± 0.225 | 0.332 ± 0.181 | 0.357 ± 0.160 | 0.305 ± 0.174 | 0.288 ± 0.108 |
| Sulfur containing compounds | ||||||||||||
| 293 | Methional | 14:02.7 | 00:01.0 | MS, LRI | 1461 | 1461 | 11.821 | 0.018 ± 0.014 c | 0.116 ± 0.047 a | 0.060 ± 0.043 b | 0.017 ± 0.005 c | 0.027 ± 0.031 bc |
| 294 | 2-(Methylthio)ethanol | 15:29.0 | 00:00.8 | S, MS, LRI | 1524 | 1531 | 9.501 | 0.261 ± 0.063 b | 0.356 ± 0.069 a | 0.290 ± 0.094 ab | 0.133 ± 0.020 c | 0.237 ± 0.103 b |
| 295 | Methionol | 19:08.8 | 00:00.9 | S, MS, LRI | 1729 | 1733 | 5.647 | 2.344 ± 0.660 bc | 4.022 ± 1.550 a | 3.056 ± 1.076 ab | 1.741 ± 0.881 c | 1.465 ± 0.676 c |
| 296 | Ethyl thiophene-2-carboxylate | 20:02.0 | 00:01.2 | MS | 1775 | - | 4.883 | 0.024 ± 0.006 a | 0.020 ± 0.003 ab | 0.018 ± 0.005 bc | 0.017 ± 0.004 bc | 0.012 ± 0.002 c |
| 297 | 4-(Methylthio)-1-butanol | 21:26.0 | 00:00.9 | MS | 1850 | - | 4.672 | 0.011 ± 0.005 bc | 0.022 ± 0.010 a | 0.018 ± 0.009 ab | 0.009 ± 0.004 c | 0.007 ± 0.003 c |
| 298 | S-(3-hydroxypropyl) thioacetate | 14:47.0 | 00:01.1 | MS | 1491 | - | 4.320 | 0.052 ± 0.015 b | 0.083 ± 0.032 a | 0.062 ± 0.016 b | 0.048 ± 0.006 b | 0.042 ± 0.020 b |
| 299 | 2-Thiophenecarboxaldehyde | 18:45.0 | 00:01.0 | S, MS, LRI | 1708 | 1701 | 3.796 | 0.039 ± 0.017 b | 0.087 ± 0.046 a | 0.063 ± 0.036 ab | 0.032 ± 0.013 b | 0.069 ± 0.020 ab |
| 300 | Ethyl methanesulfonate | 18:31.0 | 00:00.9 | MS | 1695 | - | 3.638 | 0.177 ± 0.028 a | 0.157 ± 0.037 a | 0.159 ± 0.030 a | 0.120 ± 0.023 b | 0.163 ± 0.029 a |
| 301 | Ethyl 3-(methylthio)-trans-2-propenoate | 19:41.0 | 00:01.2 | MS, LRI | 1757 | 1733 | 2.915 | 0.013 ± 0.007 b | 0.021 ± 0.008 a | 0.012 ± 0.004 b | 0.018 ± 0.003 ab | 0.010 ± 0.007 b |
| 302 | 3-(Methylthio)propyl acetate | 17:28.7 | 00:01.2 | MS, LRI | 1630 | 1627 | 2.832 | 0.589 ± 0.319 ab | 0.731 ± 0.337 a | 0.385 ± 0.305 bc | 0.427 ± 0.061 bc | 0.202 ± 0.087 c |
| 303 | Ethyl 3-(methylthio)-trans-2-propenoate | 21:33.0 | 00:01.2 | MS, LRI | 1856 | 1837 | 2.795 | 0.002 ± 0.004 b | 0.011 ± 0.006 a | 0.006 ± 0.005 ab | 0.010 ± 0.006 a | 0.005 ± 0.006 ab |
| 304 | Diethyl sulfate | 17:21.0 | 00:01.0 | MS | 1622 | - | 2.496 | 0.013 ± 0.003 | 0.010 ± 0.004 | 0.011 ± 0.003 | 0.009 ± 0.002 | 0.011 ± 0.002 |
| 305 | Ethyl thiocyanate | 21:05.2 | 00:01.2 | MS | 1831 | - | 2.399 | 0.250 ± 0.090 | 0.299 ± 0.119 | 0.279 ± 0.086 | 0.251 ± 0.073 | 0.112 ± 0.045 |
| 306 | 3-Ethoxythiophene | 14:19.0 | 00:01.2 | MS | 1472 | - | 1.656 | 0.030 ± 0.015 | 0.042 ± 0.035 | 0.054 ± 0.039 | 0.023 ± 0.005 | 0.020 ± 0.018 |
| 307 | S-[(2,5-dihydro-4-hydroxy-5-oxo-3-furanyl)methyl] ethanethioate | 23:09.7 | 00:00.8 | MS | 1942 | - | 1.376 | 0.113 ± 0.149 | 0.032 ± 0.055 | 0.048 ± 0.057 | 0.030 ± 0.024 | 0.016 ± 0.020 |
| 308 | 1-(tert-Butylsulfonyl)-2-octanol | 19:14.9 | 00:02.2 | MS | 1734 | - | 0.996 | 0.182 ± 0.125 | 0.193 ± 0.116 | 0.220 ± 0.078 | 0.160 ± 0.086 | 0.086 ± 0.078 |
| 309 | Cyclohexyl isothiocyanate | 18:17.0 | 00:01.6 | MS, LRI | 1681 | 1667 | 0.780 | 0.014 ± 0.011 | 0.017 ± 0.011 | 0.017 ± 0.009 | 0.014 ± 0.008 | 0.006 ± 0.005 |
| 310 | 3-[(2-Hydroxyethyl)thio]-1-propanol | 20:58.0 | 00:00.9 | MS | 1825 | - | 0.515 | 0.020 ± 0.004 | 0.019 ± 0.006 | 0.027 ± 0.023 | 0.029 ± 0.026 | 0.022 ± 0.009 |
| 311 | 2-Methyldihydro-3(2H)-thiophenone | 15:28.6 | 00:01.1 | MS, LRI | 1523 | 1538 | 0.510 | 1.630 ± 0.556 | 1.418 ± 0.673 | 1.130± 0.911 | 1.449 ± 0.752 | 1.235 ± 0.462 |
| Other compounds | ||||||||||||
| 312 | 2,6,10,10-Tetramethyl-1-oxaspiro[4.5]deca-3,6-diene | 15:50.0 | 00:01.9 | MS | 1541 | - | 7.995 | 0.139 ± 0.058 a | 0.025 ± 0.012 c | 0.076 ± 0.036 b | 0.054 ± 0.020 bc | 0.075 ± 0.072 bc |
| 313 | Ethylene diglycol monoethyl ether | 17:19.0 | 00:00.9 | MS, LRI | 1620 | 1622 | 5.688 | 0.081 ± 0.031 b | 0.238 ± 0.114 a | 0.175 ± 0.095 a | 0.227 ± 0.062 a | 0.261 ± 0.017 a |
| 314 | Acetic formic anhydride | 15:59.6 | 00:00.7 | MS | 1549 | - | 1.865 | 0.065 ± 0.090 | 0.159 ± 0.132 | 0.151 ± 0.102 | 0.059 ± 0.084 | 0.040 ± 0.053 |
| 315 | Crotonic anhydride | 21:25.8 | 00:01.5 | MS | 1850 | - | 1.557 | 0.077 ± 0.060 | 0.063 ± 0.042 | 0.043 ± 0.023 | 0.065 ± 0.018 | 0.020 ± 0.017 |
| 316 | 1H-indole | 31:00.2 | 00:00.9 | MS, LRI | >2100 | 2420 | 1.036 | 0.044 ± 0.012 | 0.041 ± 0.019 | 0.037 ± 0.021 | 0.027 ± 0.005 | 0.035 ± 0.026 |
| 317 | Methylsuccinic anhydride | 18:59.0 | 00:00.9 | MS | 1720 | - | 0.517 | 0.013 ± 0.021 | 0.051 ± 0.112 | 0.038 ± 0.028 | 0.029 ± 0.026 | 0.053 ± 0.009 |
ID—identification of compounds; S—retention time and mass spectrum consistent with that of the pure standard and with NIST05 mass spectra electronic library; LRI—linear retention index consistent with that found in literature; MS—mass spectra consistent with that from NIST 2.0, Wiley 8, and FFNSC 2 mass spectra electronic libraries or literature; n.i.—not identified. The compounds with only MS symbol in ID column were tentatively identified. LRIlit—linear retention index from the literature, LRIexp—linear retention index obtained experimentally. Varieties: MI—Malvazija istarska, PO—Pošip, MA—Maraština, KR—Kraljevina, SK—Škrlet. Different superscript lowercase letters in a row represent statistically significant differences between mean values at p < 0.05 obtained by one-way ANOVA and least significant difference (LSD) test.
In order to compare the techniques applied, the GC×GC-TOF-MS results for the major monoterpenols and some other compounds already quantified by GC-MS and reported in Table 1 were also reported in Table 2. It was observed that the results, in relative terms, were mostly in fair agreement. α-Terpineol was confirmed as a monoterpene and a volatile aroma compound in general with the highest discriminative power, with an F-ratio even higher than that obtained after GC-MS data elaboration. α-Terpineol was followed by limonene and linalool, as well as some other monoterpenes which were also among the most potent volatiles according to this criterion as determined by GC-MS, such as nerol, ho-trienol, 4-terpineol, and trans-β-ocimene. On the other hand, some discrepancies were observed; for example, in the case of geraniol, α-terpinolene, and geranyl ethyl ether, with a high F-ratio obtained by GC×GC-TOF-MS and a relatively low F-ratio obtained by GC-MS data elaboration. The opposite was observed for citronellol. It is possible that the discrepancies observed derived from the co-elution of the mentioned monoterpenes with particular unidentified compounds having mass spectra with ions of equal mass to those used for quantification of terpenes during GC-MS analysis, although strict measures have been taken to ensure the quality of the results.
Similar as in the case of GC-MS results (Table 1), Škrlet wines were the most abundant in monoterpenes, followed by Malvazija istarska, then Pošip, and finally Maraština and Kraljevina wines with the lowest concentrations (Table 2). Only a few exceptions were noted: Škrlet wines contained the lowest concentration of β-calacorene, while Malvazija wine was deficient in cis-Z-α-bisabolene epoxide. Although Kraljevina wine was generally poor in terpenes, several sesquiterpenes, such as cadalene, β-calacorene, and especially tentatively identified γ-dehydro-ar-himachalene, emerged as potential markers of the varietal origin of this wine.
All the other classes of compounds were confirmed to be far less efficient in differentiating the investigated monovarietal wines than terpenes, with few exceptions. The number of C13-norisoprenoids identified by the two techniques applied was similar, but their identities differed in most cases. The relative results for β-damascenone obtained by GC-MS and GC×GC-TOF-MS were in a fair agreement, with the highest concentration found in Malvazija istarska and the lowest in Škrlet wines (Table 2). A similar degree of correspondence between GC-MS and GC×GC-TOF-MS results and the corresponding F-ratios was observed for a vitispirane isomer. Kraljevina wines contained the highest concentration of tentatively identified 1,2-dihydro-1,4,6-trimethylnaphthalene.
Superiority of GC×GC-TOF-MS over GC-MS in terms of compound separation and identification was demonstrated well in the analysis of benzenoids, with a much larger number of compounds identified by the former technique. Several benzenoids were found to be relatively efficient discriminators between monovarietal wines, and some of them were exclusive differentiators for particular varieties. High ethyl benzene concentration was specific for Pošip, while 1,1′-oxybisbenzene was most abundant in Malvazija istarska wines, in both cases supported by rather high F-ratios. In addition to the highest concentration of 1,1′-oxybisbenzene, Malvazija istarska wine was characterized by most varietal markers among benzenoids, including octylbenzene, a non-identified benzenoid, azulene, 2-methylnaphthalene, and methyl 2-(benzyloxy)propanoate. Pošip was characterized by the highest ethyl benzene and trans-edulan concentration, Kraljevina was the most abundant in 6-[1-(hydroxymethyl)vinyl]-4,8a-dimethyl-1,2,4a,5,6,7,8,8a-octahydro-2-naphthalenol, while Škrlet wine was the richest in m-methoxyanisole and α,α-dimethylbenzenemethanol (Table 2).
No significant differences were found between the concentrations of hydrocarbons, while aldehydes also turned out to be poor varietal differentiators, with significant differences found only for decanal (Table 2). On the other hand, several ketones were found useful for this purpose: the highest concentration of 2-undecanone and 3-undecanone was specific for Malvazija istarska, 1,4,7,10,13-pentaoxacyclononadecane-14,19-dione and cyclohexylideneacetone were characteristic for Škrlet, while the lowest concentration of isophorone was found in Maraština wines.
4-Methyl-1-heptanol was the most useful among alcohols in differentiating monovarietal wines with a rather high F-ratio (Table 2). It was found in the highest concentration in Škrlet, followed by Malvazija istarska wines, while the other wines contained lower concentrations. The results for cis-3-hexen-1-ol were in accordance with those obtained by GC-MS, with the highest concentration found in Dalmatian Pošip and Maraština wines. 3-Octanol and 1-octen-3-ol were exclusive markers for Pošip, 2-decanol for Škrlet, while the lowest concentration of an isomer of 2-penten-1-ol was characteristic for Kraljevina wine. F-ratios determined for fatty acids were relatively low and significant differences were found only for five of them.
A very large number of minor esters was identified by GC×GC-TOF-MS analysis (Table 2). In accordance with the GC-MS data, the concentrations of the majority of esters of aliphatic higher alcohols and fatty acids were the highest in Malvazija istarska wines. Despite the thesis that precursor concentrations do not significantly determine the concentrations of acetate esters formed by Saccharomyces cerevisiae [60], the highest concentration of cis-3-hexen-1-yl acetate corresponded to the highest concentration of its precursor, cis-3-hexen-1-ol, found in Pošip wine. Pošip wine was the most abundant in particular esters of ethanol and hydroxyl keto acids, such as diethyl glutarate and ethyl pyruvate. Although without a statistically significant difference, the concentrations of the related esters, such as ethyl lactate and diethyl succinate, determined by GC-MS, also had a tendency to be higher in Pošip wines.
Pošip wines contained the highest concentration of volatile phenols, such as 2-methoxyphenol and 4-vinylguaiacol. Significant differences were found for particular furanoids and lactones. A number of sulfur containing compounds was identified, with many of them found in the highest concentration in Pošip wines, some with relatively high F-ratios, such as methional. Kraljevina and Škrlet wines were generally the least abundant in these compounds (Table 2).
3.3. Multivariate Statistical Analysis
PCA allowed a good separation of the investigated monovarietal wines according to variety when applied on a dataset reduced to 40 variables with the highest F-values, obtained by both GC-MS and GC×GC-TOF-MS analysis. Monovarietal wines were clearly separated from each other in two-dimensional space despite a relatively high number of varieties (Figure 1). Škrlet wine was clearly differentiated from the others along the direction of PC1 and was characterized by higher amounts of terpenes. A part of Malvazija istarska wines also gravitated towards higher positive PC1 values, but the wines of this variety were also separated from the others along the direction of PC2, mostly due to higher concentrations of particular esters with positive PC2 values. Volatile aroma compounds located in the second quadrant of Cartesian system with negative PC1 and positive PC2 coordinates, 2,3-dihydro-1,1,5,6-tetramethyl-1H-indene and γ-dehydro-ar-himachalene, contributed most to the separation of Kraljevina wines, while the location of Pošip wines was obviously conditioned by the loadings of cis-3-hexen-1-ol, vitispirane II, ethyl benzoate, methional, cis-3-hexen-1-yl acetate, 2-phenethyl acetate, and 2-(methylthio)ethanol. Maraština wines were apparently not linked to any particular compound class, probably due to lower concentrations of the 40 volatile compounds used for PCA.
Figure 1.
(a) Separation of Croatian monovarietal wines according to variety in two-dimensional space defined by the first two principal components, PC1 and PC2; (b) Factor loadings of selected variables (40 volatile aroma compounds with the highest F-ratios), as determined by gas chromatography mass spectrometry (GC-MS) and two-dimensional gas chromatography with time-of-flight mass spectrometry (GC×GC-TOF-MS) analysis, on PC1 and PC2.
Hierarchical clustering analysis according to variety, performed using the amounts of the 60 volatile aroma compounds with the highest F-ratio, confirmed that each monovarietal wine had a distinct volatile profile (Figure 2). Most of the conclusions were similar to those obtained by the PCA. Škrlet and Malvazija Istarska wines were clearly separated from each other mostly due to higher concentrations of particular esters in the latter, but were clustered together by high terpene concentrations. The generated heatmap probably offered the clearest insight into the intra-varietal diversity of particular wines, especially Malvazija with two evident clusters with different terpene content. Pošip formed a distinct cluster mostly due to high concentrations of particular compounds from several classes, some of them already mentioned in the PCA, including vitispirane II, trans-edulan, methional, 2-phenyletahnol, cis-3-hexen-1-ol and its acetate, ethyl benzoate, 2-heptanol, 2-phenethyl acetate, ethyl cinnamate, and others. Kraljevina wines were clearly the least abundant in the majority of the 60 pre-selected compounds, except for γ-dehydro-ar-himachalene, 1,2-dihydro-1,4,6-trimethylnaphthalene and particular benzenoids, which were confirmed as its markers.
Figure 2.
Hierarchical clustering analysis performed using volatile aroma compound profiles of Croatian monovarietal wines obtained by GC-MS and GC×GC-TOF-MS analysis. The heatmap was generated using 60 most significant compounds (the highest F-ratios). The rows in the heatmap represent compounds and the columns indicate samples. Compounds are designated by numbers which correspond to those in Table 1 (GC, i.e., GC-MS) or in Table 2 (GCGC, i.e., GC×GC-TOF-MS). The colors of heatmap cells indicate the abundance of compounds across different samples. The color gradient, ranging from dark blue through white to dark red, represents low, middle, and high abundance of a compound.
SLDA was applied on a dataset reduced to 60 most significant volatile aroma compounds according to F-ratio from both GC-MS and GC×GC-TOF-MS original datasets. All the monovarietal wines were classified correctly according to variety by this model, and 24 most significant variables were extracted (Figure 3), with rather high squared Mahalanobis distances from group centroids. A 100% correct classification was obtained after including only seven variables. α-Terpineol was confirmed once again as the most powerful varietal marker, since the SLDA model classified correctly 68.75% of all the wines and 100.00% of Škrlet wines by using only this variable. After including β-pinene and ethyl benzoate the total percentage of correctly classified wines increased to 93.75%. For achieving a 100.00% correct classification, 1,1′-oxybisbenzene, γ-dehydro-ar-himachalene, vitispirane II, and 2,6,10,10-tetramethyl-1-oxaspiro[4.5]deca-3,6-diene were included in the SLDA model. The following 17 volatile aroma compounds were also included: 2-phenethyl acetate, isophorone, monoterpenyl acetate (n.i.; m/z 93, 69, 121), 2,3-dihydro-1,1,5,6-tetramethyl-1H-indene II, cis-3-hexen-1-ol, methyl hexanoate, trans-rose oxide, methyl decanoate, cis-3-hexen-1-yl acetate, monoterpene (n.i.; m/z 93, 69, 41), β-myrcene, limonene, 3-methyl-2(5H)-furanone, 2-phenylethanol, 1,2-dihydro-1,4,6-trimethylnaphthalene, nerol, and nerol oxide.
Figure 3.
Separation of Croatian monovarietal wines according to variety defined by the first three discriminant functions (roots) obtained by forward stepwise discriminant analysis (SLDA) on the basis of volatile aroma compound composition determined by GC-MS and GC×GC-TOF-MS analysis. (a) root 1 vs root 2; (b) root 1 vs root 3; (c) root 2 vs root 3.
Apparently, SLDA has extracted volatile aroma compounds which were most useful for the differentiation of the five investigated monovarietal wines between each other, which only partly coincided with the compounds with the highest F-ratios obtained by ANOVA. Monoterpenes had a key role again, especially α-terpineol. The ability of the SLDA model to predict a correct variety was checked by “leave-one-out” cross-validation, where each wine sample was excluded and classified by the functions derived from all the other wine samples. The correct prediction rate achieved was 100.00%.
To compare the usefulness of the information contained in the composition of terpenes alone obtained by GC-MS and GC×GC-TOF-MS analysis for differentiating monovarietal wines, SLDA was applied separately on the two datasets containing 20 and 31 terpenes, respectively, found significant by ANOVA. Both GC-MS and GC×GC-TOF-MS dataset based models succeeded in achieving 100.00% correct classification (Figure 4). α-Terpineol was again confirmed as a key differentiator, since both models included it as the first, which classified correctly 59.38% and 68.75% monovarietal wines, respectively. For achieving 100.00% correct classification, the GC-MS model further included trans-ocimene, cis-linalool furan oxide, β-pinene, citronellol, trans-nerolidol, ho-trienol, trans-rose oxide, and limonene, while the GC×GC-TOF-MS model extracted γ-dehydro-ar-himachalene, ho-trienol, nerol, o-cymene, isogeraniol, a non-identified sesquiterpene (n.i.; m/z 119, 93, 69), neryl ethyl ether, and cis-α-ocimene. The classification efficacy of the models was improved by including further eight and nine terpenes, respectively. The GC×GC-TOF-MS model exhibited a superior efficacy judging from the degree of the overlapping of the corresponding 95% confidence areas, as well as higher squared Mahalanobis distances on the average, especially for Škrlet wines.
Figure 4.
Separation of Croatian monovarietal wines according to variety defined by the first three discriminant functions (roots) obtained by forward stepwise discriminant analysis (SLDA) on the basis of volatile terpene composition determined by GC-MS (a–c) and GC×GC-TOF-MS (d–f) analysis.
The volatile aroma compounds which were found to be most useful for the differentiation of the investigated wines in this study were only partly in accordance with the ones highlighted in previous studies which applied a similar multivariate statistical approach. For example, Welke et al. [29] characterized and differentiated wines from Chardonnay, Sauvignon Blanc, Pinot Noir, Merlot, and Cabernet Sauvignon based on volatile aroma composition obtained by GC×GC-TOF-MS analysis and extracted the following 12 volatile compounds as the most useful for their differentiation: diethyl succinate, 2,3-butanediol, nerol, 3-penten-2-one, diethyl malonate, β-santalol, ethyl 9-decenoate, alcohol-C9, 4-carene, tetrahydro-2(2H)-pyranone, dihydro-2(3H)-thiophenone, and 3-methyl-2(5H)-furanone. It is probable that the main reason for such discrepancy between this and the study from Welke et al. [29] was the fact that the mentioned authors mutually compared wines from white and red varieties, which greatly differ with respect to the production technology, which, besides variety, certainly greatly contributed to the differences between wines. Welke et al. [29] also obtained a SLDA model that differentiated wines according to variety with a 100% correct recognition ability, while some other authors who applied conventional GC-MS for the same purpose, such as Zhang et al. [61] and Câmara, Alves and Marques [14], did not succeed completely. Fabani, Ravera, and Wunderlin [15] obtained a 100% correct discrimination among Syrah, Malbec, and Bonarda red wines by the application of SLDA on GC-MS data with ethyl hexanoate, ethyl octanoate, 1-hexanol, benzyl alcohol, and isoamyl acetate as the most useful differentiators. Terpenes were not analyzed. Ziółkowska, Wąsowicz, and Jeleń [19] obtained a relatively good differentiation of red wines, with the ability of the LDA model to correctly classify and predict their varietal origin based on HS-SPME/GC-MS data of 95%, while the model built for white wines was not that successful. The compounds most useful for the differentiation of white wines (Chardonnay, Sauvignon Blanc, and Muscat) were isoamyl acetate, furfural, ethyl octanoate, ethyl decanoate, and ethyl dodecanoate, while red wines (Cabernet Sauvignon and Merlot) were differentiated mainly by 1-hexanol, ethyl decanoate, and 2-phenylethanol. It should be noted that the samples of the same variety were collected across several countries, which was certainly a factor that introduced large variability.
4. Conclusions
HS-SPME/GC×GC-TOF-MS analysis, alone or combined with conventional HS-SPME/GC-MS, was shown to be an excellent analytical tool for differentiation of wines according to variety based on volatile aroma compound composition. It has also been proven that the additional separation efficiency enabled by the second chromatographic column in GC×GC-TOF-MS analysis was crucial for the separation and identification of a very large number of volatile compounds, which would otherwise remain undetected by conventional GC-MS. This feature provided additional in-depth volatile profile information which was exploited for highly efficient white wine varietal differentiation. Such an outcome can be considered even more successful knowing that the number of varieties was relatively high while that of wine samples of each variety was relatively small, and that the investigated wines were characterized by high intra-varietal heterogeneity in terms of micro-locations and grape cultivation and winemaking parameters. The results of this study confirmed the unmatched power of monoterpenes to discriminate wines according to variety, which was robust enough to be captured by uni- and multivariate statistics based on both GC-MS and GC×GC-TOF-MS analysis data separately.
Acknowledgments
The authors would like to thank wine producers from Croatia for donating wine samples, Irena Budić-Leto, Sanja Radeka, and the panel for sensory analysis of wine from the Institute of Agriculture and Tourism in Poreč, Croatia, for assistance in collecting and selection of wine samples, Ivana Puhelek for assistance in collecting wine samples, and Ivana Horvat for technical assistance.
Supplementary Materials
The following are available online at https://www.mdpi.com/2304-8158/9/12/1787/s1, Figure S1: Example of a contour plot obtained for Malvazija istarska monovarietal wine using HS-SPME/GC×GC-TOF-MS. Colored areas represent more abundant volatile aroma compounds and black dots represent less abundant and trace volatile aroma compounds, Table S1: Physico-chemical parameters in in Croatian monovarietal wines, Table S2: Concentrations (μg/L) of volatile aroma compounds found in individual Croatian monovarietal wines after headspace solid-phase microextraction followed by gas chromatography-mass spectrometry (HS-SPME/GC–MS) sorted by compound class, Table S3: Concentrations (μg/L relative to internal standard 2-octanol) of volatile aroma compounds found in individual Croatian monovarietal wines obtained by headspace solid-phase microextraction combined with comprehensive two-dimensional gas chromatography-mass spectrometry with time-of-flight mass spectrometric detection (HS-SPME/GC×GC-TOF-MS) sorted by compound class.
Author Contributions
Conceptualization, I.L.; methodology, I.L. and U.V.; formal analysis, I.L. and S.C.; investigation, I.L. and S.C.; resources, I.L. and U.V.; data curation, I.L. and U.V.; writing—original draft preparation, I.L.; writing—review and editing, I.L. and U.V.; supervision, I.L. and U.V.; project administration, I.L. and U.V.; funding acquisition, I.L. and U.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Croatian Science Foundation grant number UIP-2014-09-1194 and by the ADP 2017 project funded by the Autonomous Province of Trento.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ribéreau-Gayon P., Glories Y., Maujean A., Dubourdieu D. Handbook of Enology, The Chemistry of Wine: Stabilization and Treatments. Volume 2. John Wiley & Sons Ltd.; Chichester, UK: 2000. [Google Scholar]
- 2.Rapp A., Mandery H. Wine aroma. Experientia. 1986;42:873–884. doi: 10.1007/BF01941764. [DOI] [Google Scholar]
- 3.Ebeler S.E. Analytical chemistry: Unlocking the secrets of wine flavor. Food Rev. Int. 2001;17:45–64. doi: 10.1081/FRI-100000517. [DOI] [Google Scholar]
- 4.Ilc T., Werck-Reichhart D., Navrot N. Meta-Analysis of the Core Aroma Components of Grape and Wine Aroma. Front. Plant Sci. 2016;7:1472:1–1472:15. doi: 10.3389/fpls.2016.01472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villano C., Lisanti M.T., Gambuti A., Vecchio R., Moio L., Frusciante L., Aversano R., Carputo D. Wine varietal authentication based on phenolics, volatiles and DNA markers: State of the art, perspectives and drawbacks. Food Control. 2017;80:1–10. doi: 10.1016/j.foodcont.2017.04.020. [DOI] [Google Scholar]
- 6.Koundouras S., Marinos V., Gkoulioti A., Kotseridis Y., van Leeuwen C.J. Influence of vineyard location and vine water status on fruit maturation of nonirrigated cv. Agiorgitiko (Vitis vinifera L.). Effects on wine phenolic and aroma components. J. Agric. Food Chem. 2006;54:5077–5086. doi: 10.1021/jf0605446. [DOI] [PubMed] [Google Scholar]
- 7.Sabon I., de Revel G., Kotseridis Y., Bertrand A. Determination of volatile compounds in Grenache wines in relation with different terroirs in the Rhone Valley. J. Agric. Food Chem. 2002;50:6341–6345. doi: 10.1021/jf025611k. [DOI] [PubMed] [Google Scholar]
- 8.Yue X., Ma X., Tang Y., Wang Y., Wu B., Jiao X., Zhang Z., Ju Y. Effect of cluster zone leaf removal on monoterpene profiles of Sauvignon Blanc grapes and wines. Food Res. Int. 2020;131:109028:1–109028:11. doi: 10.1016/j.foodres.2020.109028. [DOI] [PubMed] [Google Scholar]
- 9.Šuklje K., Carlin S., Stanstrup J., Antalick G., Blackman J.W., Meeks C., Deloire A., Schmidtke L.M., Vrhovsek U. Unravelling wine volatile evolution during shiraz grape ripening by untargeted HS-SPME-GC×GC-TOFMS. Food Chem. 2019;277:753–765. doi: 10.1016/j.foodchem.2018.10.135. [DOI] [PubMed] [Google Scholar]
- 10.Falqué E., Fernández E., Dubourdieu D. Differentiation of white wines by their aromatic index. Talanta. 2001;54:271–281. doi: 10.1016/S0039-9140(00)00641-X. [DOI] [PubMed] [Google Scholar]
- 11.Martínez-Pinilla O., Guadalupe Z., Ayestarán B., Pérez-Magariño S., Ortega-Heras M. Characterization of volatile compounds and olfactory profile of red minority varietal wines from La Rioja. J. Sci. Food Agric. 2013;93:3720–3729. doi: 10.1002/jsfa.6211. [DOI] [PubMed] [Google Scholar]
- 12.Barbará J.A., Primieri Nicolli K., Souza-Silva É.A., Telles Biasoto A.C., Welke J.E., Alcaraz Zini C. Volatile profile and aroma potential of tropical Syrah wines elaborated in different maturation and maceration times using comprehensive two-dimensional gas chromatography and olfactometry. Food Chem. 2020;308:125552. doi: 10.1016/j.foodchem.2019.125552. [DOI] [PubMed] [Google Scholar]
- 13.Samoticha J., Wojdyło A., Chmielewska J., Nofer J. Effect of Different Yeast Strains and Temperature of Fermentation on Basic Enological Parameters, Polyphenols and Volatile Compounds of Aurore White Wine. Foods. 2019;8:599. doi: 10.3390/foods8120599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Câmara J.S., Alves M.A., Marques J.C. Multivariate analysis for the classification and differentiation of Madeira wines according to main grape varieties. Talanta. 2006;68:1512–1521. doi: 10.1016/j.talanta.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Fabani M.P., Ravera M.J.A., Wunderlin D.A. Markers of typical red wine varieties from the Valley of Tulum (San Juan-Argentina) based on VOCs profile and chemometrics. Food Chem. 2013;141:1055–1062. doi: 10.1016/j.foodchem.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 16.Gómez García-Carpintero E., Sánchez-Palomo E., Gómez Gallego M.A., González-Viñas M.A. Free and bound volatile compounds as markers of aromatic typicalness of Moravia Dulce, Rojal and Tortosí red wines. Food Chem. 2012;131:90–98. doi: 10.1016/j.foodchem.2011.08.035. [DOI] [Google Scholar]
- 17.Rocha S.M., Coutinho P., Coelho E., Barros A.S., Delgadillo I., Coimbra M.A. Relationships between the varietal volatile composition of the musts and white wine aroma quality. A four year feasibility study. LWT Food Sci. Technol. 2010;43:1508–1516. doi: 10.1016/j.lwt.2010.06.007. [DOI] [Google Scholar]
- 18.Valentin L., Barroso L.P., Barbosa R.M., de Paulo G.A., Castro I.A. Chemical typicality of South American red wines classified according to their volatile and phenolic compounds using multivariate analysis. Food Chem. 2020;302:125340:1–125340:8. doi: 10.1016/j.foodchem.2019.125340. [DOI] [PubMed] [Google Scholar]
- 19.Ziółkowska A., Wąsowicz E., Jeleń H.H. Differentiation of wines according to grape variety and geographical origin based on volatiles profiling using SPME-MS and SPME-GC/MS methods. Food Chem. 2016;213:714–720. doi: 10.1016/j.foodchem.2016.06.120. [DOI] [PubMed] [Google Scholar]
- 20.Welke J.E., Manfroi V., Zanus M., Lazarotto M., Alcaraz Zini C. Characterization of the volatile profile of Brazilian Merlot wines through comprehensive two dimensional gas chromatography time-of-flight mass spectrometric detection. J. Chromatogr. A. 2012;1226:124–139. doi: 10.1016/j.chroma.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Weldegergis B.T., de Villiers A., McNeish C., Seethapathy S., Mostafa A., Górecki T., Crouch A.M. Characterisation of volatile components of Pinotage wines using comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC×GC–TOF-MS) Food Chem. 2011;129:188–199. doi: 10.1016/j.foodchem.2010.11.157. [DOI] [Google Scholar]
- 22.Tranchida P.Q., Donato P., Cacciola F., Beccaria M., Dugo P., Mondello L. Potential of comprehensive chromatography in food analysis. TrAC Trend. Anal. Chem. 2013;52:186–205. doi: 10.1016/j.trac.2013.07.008. [DOI] [Google Scholar]
- 23.Purcaro G., Cordero C., Liberto E., Bicchi C., Conte L.S. Toward a definition of blueprint of virgin olive oil by comprehensive two-dimensional gas chromatography. J. Chromatogr. A. 2014;1334:101–111. doi: 10.1016/j.chroma.2014.01.067. [DOI] [PubMed] [Google Scholar]
- 24.Robinson A.L., Boss P.K., Heymann H., Solomon P.S., Trengove R.D. Influence of Yeast Strain, Canopy Management, and Site on the Volatile Composition and Sensory Attributes of Cabernet Sauvignon Wines from Western Australia. J. Agric. Food Chem. 2011;59:3273–3284. doi: 10.1021/jf104324d. [DOI] [PubMed] [Google Scholar]
- 25.Ryan D., Watkins P., Smith J., Allen M., Marriott P. Analysis of methoxypyrazines in wine using headspace solid phase microextraction with isotope dilution and comprehensive two-dimensional gas chromatography. J. Sep. Sci. 2005;28:1075–1082. doi: 10.1002/jssc.200500097. [DOI] [PubMed] [Google Scholar]
- 26.Šuklje K., Carlin S., Antalick G., Blackman J.W., Deloire A., Vrhovsek U., Schmidtke L.M. Regional Discrimination of Australian Shiraz Wine Volatome by Two-Dimensional Gas Chromatography Coupled to Time-of-Flight Mass Spectrometry. J. Agric. Food Chem. 2019;67:10273–10284. doi: 10.1021/acs.jafc.9b03563. [DOI] [PubMed] [Google Scholar]
- 27.Welke J.E., Zanus M., Lazzarotto M., Alcaraz Zini C. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res. Int. 2014;59:85–99. doi: 10.1016/j.foodres.2014.02.002. [DOI] [Google Scholar]
- 28.Carlin S., Vrhovsek U., Lonardi A., Landi L., Mattivi F. Aromatic complexity in Verdicchio wines: A case study. OENO One. 2019;4:597–610. doi: 10.20870/oeno-one.2019.53.4.2396. [DOI] [Google Scholar]
- 29.Welke J.E., Manfroi V., Zanus M., Lazzarotto M., Alcaraz Zini C. Differentiation of wines according to grape variety using multivariate analysis of comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometric detection dana. Food Chem. 2013;141:3897–3905. doi: 10.1016/j.foodchem.2013.06.100. [DOI] [PubMed] [Google Scholar]
- 30.Bubola M., Lukić I., Radeka S., Sivilotti P., Grozić K., Vanzo A., Bavčar D., Lisjak K. Enhancement of Istrian Malvasia wine aroma and hydroxycinnamate composition by hand and mechanical leaf removal. J. Sci. Food Agric. 2019;99:904–914. doi: 10.1002/jsfa.9262. [DOI] [PubMed] [Google Scholar]
- 31.Carlin S., Vrhovsek U., Franceschi P., Lotti C., Bontempo L., Camin F., Toubiana D., Zottele F., Toller G., Fait A., et al. Regional features of northern Italian sparkling wines, identified using solid-phase micro extraction and comprehensive two-dimensional gas chromatography coupled with time-of-flight mass spectrometry. Food Chem. 2016;208:68–80. doi: 10.1016/j.foodchem.2016.03.112. [DOI] [PubMed] [Google Scholar]
- 32.Beckner Whitener M.E., Stanstrup J., Panzeri V., Carlin S., Divol B., Toit M., Vrhovsek U. Untangling the wine metabolome by combining untargeted SPME–GCxGC-TOF-MS and sensory analysis to profile Sauvignon blanc co-fermented with seven different yeasts. Metabolomics. 2016;12:53:1–53:25. doi: 10.1007/s11306-016-0962-4. [DOI] [Google Scholar]
- 33.Xia J., Sinelnikov I., Han B., Wishart D.S. MetaboAnalyst 3.0 – Making metabolomics more meaningful. Nucleic Acids Res. 2015;43:W251–W257. doi: 10.1093/nar/gkv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Black C.A., Parker M., Siebert T.E., Capone D.L., Francis I.L. Terpenoids and their role in wine flavour: Recent advances. Aust. J. Grape Wine R. 2015;21:582–600. doi: 10.1111/ajgw.12186. [DOI] [Google Scholar]
- 35.Ribéreau-Gayon P., Glories Y., Maujean A., Dubourdieu D. Handbook of Enology, The Chemistry of Wine: Stabilization and Treatments. 2nd ed. Volume 2. John Wiley & Sons; Chichester, UK: 2006. pp. 205–230. [Google Scholar]
- 36.Mateo J.J., Jiménez M. Monoterpenes in grape juice and wines. J. Chromatogr. A. 2000;881:557–567. doi: 10.1016/S0021-9673(99)01342-4. [DOI] [PubMed] [Google Scholar]
- 37.Guth H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997;45:3027–3032. doi: 10.1021/jf970280a. [DOI] [Google Scholar]
- 38.Battilana J., Emanuelli F., Gambino G., Gribaudo I., Gasperi F., Boss P.K., Grando M.S. Functional effect of grapevine 1-deoxy-D-xylulose 5-phosphate synthase substitution K284N on Muscat flavour formation. J. Exp. Bot. 2011;62:5497–5508. doi: 10.1093/jxb/err231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duchêne E., Legras J.L., Karst F., Merdinoglu D., Claudel P., Jaegli N., Pelsy F. Variation of linalool and geraniol content within two pairs of aromatic and non-aromatic grapevine clones. Aust. J. Grape Wine R. 2009;15:120–130. doi: 10.1111/j.1755-0238.2008.00039.x. [DOI] [Google Scholar]
- 40.Skinkis P.A., Bordelon B.P., Wood K. Comparison of Monoterpene Constituents in Traminette, Gewürztraminer, and Riesling Winegrapes. Am. J. Enol. Vitic. 2008;59:440–445. [Google Scholar]
- 41.Song M., Fuentes C., Loos A., Tomasino E. Free Monoterpene Isomer Profiles of Vitis vinifera L. cv. White Wines. Foods. 2018;7:27. doi: 10.3390/foods7020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Versini G., Orriols I., Dalla Serra A. Aroma components of Galician Albarińo, Loureira and Godello wines. Vitis. 1994;33:165–170. doi: 10.5073/vitis.1994.33.165-170. [DOI] [Google Scholar]
- 43.Lukić I., Horvat I. Differentiation of Commercial PDO Wines Produced in Istria (Croatia) According to Variety and Harvest Year Based on HS-SPME-GC/MS Volatile Aroma Compound Profiling. Food Technol. Biotechnol. 2017;55:95–108. doi: 10.17113/ftb.55.01.17.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Câmara J.S., Alves M.A., Carlos Marques J.C. Classification of Boal, Malvazia, Sercial and Verdelho wines based on terpenoid patterns. Food Chem. 2007;101:475–484. doi: 10.1016/j.foodchem.2006.02.004. [DOI] [Google Scholar]
- 45.Falqué E., Fernández E., Dubourdieu D. Volatile Components of Loureira, Dona Branca, and Treixadura Wines. J. Agric. Food Chem. 2002;50:538–543. doi: 10.1021/jf010631s. [DOI] [PubMed] [Google Scholar]
- 46.Márquez R., Castro R., Natera R., García-Barroso C. Characterisation of the volatile fraction of Andalusian sweet wines. Eur. Food. Res. Technol. 2008;226:1479–1484. doi: 10.1007/s00217-007-0679-8. [DOI] [Google Scholar]
- 47.Radeka S., Herjavec S., Peršurić Đ., Lukić I., Sladonja B. Effect of different maceration treatments on free and bound varietal aroma compounds in wine of Vitis vinifera L. cv. Malvazija istarska bijela. Food Technol. Biotechnol. 2008;46:86–92. [Google Scholar]
- 48.Lukić I., Jedrejčić N., Kovačević Ganić K., Staver M., Peršurić Đ. Phenolic and aroma composition of white wines produced by prolonged maceration and maturation in wooden barrels. Food Technol. Biotechnol. 2015;53:407–418. doi: 10.17113/ftb.53.04.15.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lukić I., Horvat I., Radeka S., Damijanić K., Staver M. Effect of different levels of skin disruption and contact with oxygen during grape processing on phenols, volatile aromas, and sensory caracteristics of white wine. J. Food Process. Preserv. 2019;43:e13969:1–e13969:12. doi: 10.1111/jfpp.13969. [DOI] [Google Scholar]
- 50.González-Barreiro C., Rial-Otero R., Cancho-Grande B., Simal-Gándara J. Wine aroma compounds in grapes: A critical review. Crit. Rev. Food Sci. 2013;55:202–228. doi: 10.1080/10408398.2011.650336. [DOI] [PubMed] [Google Scholar]
- 51.Tonietto J., Carbonneau A. A multicriteria climatic classification system for grape-growing regions worldwide. Agr. Forest Meteorol. 2004;124:81–97. doi: 10.1016/j.agrformet.2003.06.001. [DOI] [Google Scholar]
- 52.Belancic A., Agosin E., Ibacache A., Bordeu E., Baumes R., Razungles A., Bayonove C. Influence of sun exposure on the aromatic composition of chilean Muscat grape cultivars Moscatel de Alejandria and Moscatel rosada. Am. J. Enol. Vitic. 1997;48:181–186. [Google Scholar]
- 53.Mendes-Pinto M.M. Carotenoid breakdown products - the norisoprenoids - in wine aroma. Arch. Biochem. Biophys. 2009;483:236–245. doi: 10.1016/j.abb.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 54.Marais J., van Wyk C.J., Rapp A. Effect of sunlight and shade on norisoprenoids levels in maturing Weisser Riesling and Chenin blanc grapes and Weisser Riesling wines. S. Afr. J. Enol. Vitic. 1992;13:23–32. doi: 10.21548/13-1-2191. [DOI] [Google Scholar]
- 55.Oliveira J.M., Faria M., Sá F., Barros F., Araújo I.M. C6-alcohols as varietal markers for assessment of wine origin. Anal. Chim. Acta. 2006;563:300–309. doi: 10.1016/j.aca.2005.12.029. [DOI] [Google Scholar]
- 56.Ugliano M., Henschke P.A. Yeasts and wine flavour. In: Moreno-Arribas M.V., Polo M.C., editors. Wine Chemistry and Biochemistry. Springer; New York, NY, USA: 2009. pp. 313–392. [Google Scholar]
- 57.Louw L., Tredoux A.G.J., Van Rensburg P., Kidd M., Naes T., Nieuwoudt H.H. Fermentation derived aroma compounds in varietal young wines from South Africa. S. Afr. J. Enol. Vitic. 2010;31:213–225. doi: 10.21548/31-2-1418. [DOI] [Google Scholar]
- 58.Ferreira V., López R., Cacho J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2002;80:1659–1667. doi: 10.1002/1097-0010(20000901)80:11<1659::AID-JSFA693>3.0.CO;2-6. [DOI] [Google Scholar]
- 59.Schreier P., Jennings W.G. Flavor composition of wines: A review. Crit. Rev. Food Sci. Nutr. 1979;12:59–111. doi: 10.1080/10408397909527273. [DOI] [PubMed] [Google Scholar]
- 60.Verstrepen K.J., Van Laere S.D.M., Vanderhaegen B.M.P., Derdelinckx G., Dufour J.-P., Pretorius I.S., Winderickx J., Thevelein J.M., Delvaux F.R. Expression levels of the yeast alcohol acetyltransferase genes ATF1, Lg-ATF1, and ATF2 control the formation of a broad range of volatile esters. Appl. Environ. Microbiol. 2003;69:5228–5237. doi: 10.1128/AEM.69.9.5228-5237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang J., Li L., Gao N., Wang D., Gao Q., Jiang S. Feature extraction and selection from volatile compounds for analytical classification of Chinese red wines from different varieties. Anal. Chim. Acta. 2010;662:137–142. doi: 10.1016/j.aca.2009.12.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.