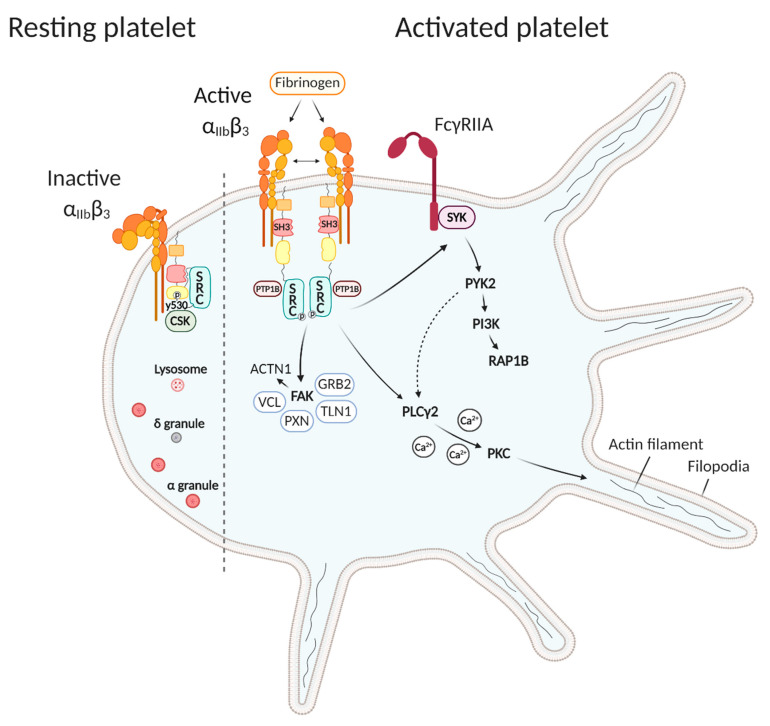
Figure 2.
The role of SRC kinase during platelet activation. Left side: In circulating resting platelets, SRC is maintained in an inactive closed conformation, mediated through Y530 phosphorylation by C-terminal SRC kinase (CSK), and is coupled to the β subunit of integrin αIIbβ3. Right side: Fibrinogen binding initiates integrin αIIbβ3 dimerization and platelet activation. After dephosphorylation of Y530 by protein-tyrosine phosphatases (PTP) and (auto)phosphorylation of Y419, SRC becomes active and initiates downstream outside-in signaling. SRC activates the focal adhesion kinase FAK, that in turn activates α-actinin (ACTN1) or recruits new binding partners such as GRB2, talin (TLN1), paxillin (PXN), and vinculin (VCL), which mobilize FAK to focal adhesions. SRC can also phosphorylate PLCγ2, which plays an important role in the formation of filopodia and lamellipodia during platelet spreading by mediating calcium signaling and PKC activity. Additionally, SRC can phosphorylate tyrosine kinase SYK, coupled to the FcγRIIa receptor, that in turn phosphorylates tyrosine kinase PYK2, which further activates PLCγ2 or connects the outside-in signaling pathway with class I phosphoinositide 3-kinase (PI3K) to mediate thrombus stability via the small GTPase RAP1B.