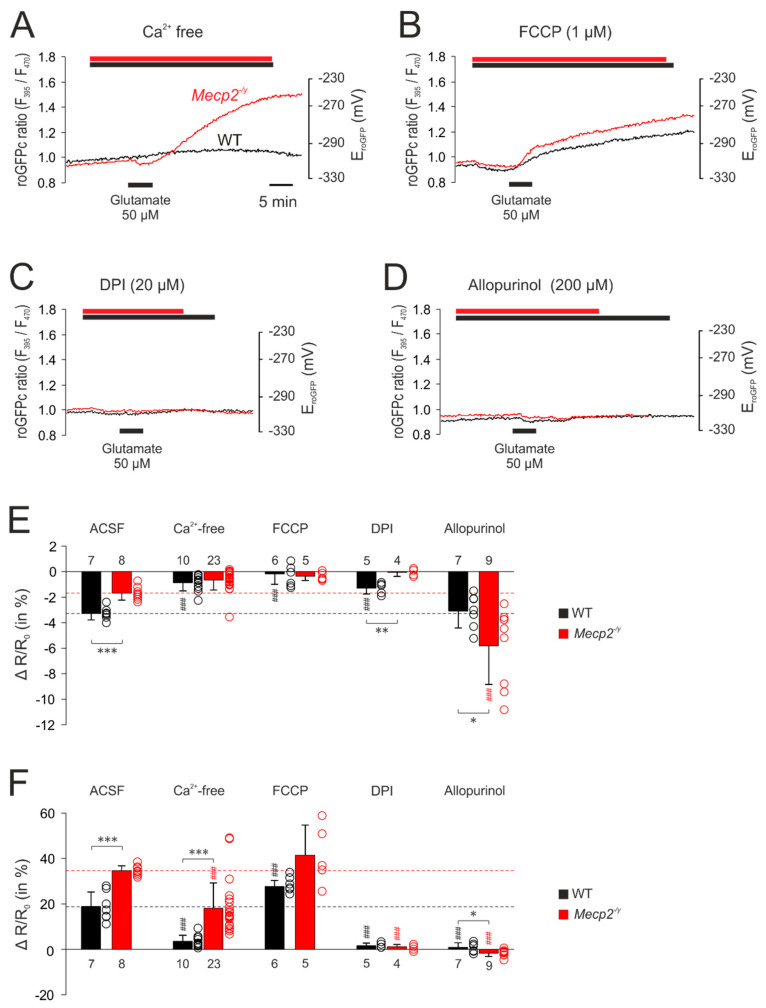
Figure 7.
Mitochondrial and extra-mitochondrial sources contribute to the glutamate-mediated oxidation of roGFPc. (A) Withdrawal of extracellular Ca2+ markedly depressed the glutamate-mediated oxidation of roGFPc in WT neurons and partly reduced it in Mecp2−/y neurons. (B) The uncoupling agent FCCP by itself mediated a slight reduction of roGFPc. Subsequent glutamate application induced a more intense oxidation of roGFPc in WT neurons; in Mecp2−/y neurons, the glutamate-mediated oxidation tended to increase. (C) Blockade of NADPH oxidase by DPI almost abolished the glutamate-mediated oxidizing responses in both genotypes. (D) Inhibition of xanthine oxidase by allopurinol severely depressed the glutamate-mediated roGFPc oxidation in both, WT and Mecp2−/y neurons. (E) Modulation of the glutamate-mediated initial reducing shift by the various treatments assessed. Plotted are the normalized changes in the roGFP ratio (ΔR/Ro), and significant differences among genotypes in this and the following panel are indicated by asterisks (* p < 0.05, ** p < 0.01, *** p < 0.001; unpaired t-test/Mann–Whitney rank sum test). Significant differences as compared to the control responses of the given genotype (ACSF) are indicated by cross-hatches (### p < 0.001; ANOVA, Holm–Sidak test). Dot plots indicate the scatter of the respective data. (F) Modulation of the glutamate-induced oxidization of roGFPc. Note that most intense antagonistic effects were observed upon inhibition of NADPH-oxidase and xanthine oxidase.