Abstract
Cerium dioxide nanomaterials (CeO2 NMs) are widely used in nano-based diesel additives to decrease the emission of toxic compounds, but they have been shown to increase the emission of ultrafine particles as well as the amount of released Ce. The Organization for Economic Cooperation and Development included CeO2 NMs in the priority list of nanomaterials that require urgent evaluation, and the potential hazard of aged CeO2 NM exposure remains unexplored. Herein, human and rat sperm cells were exposed in vitro to a CeO2 NM-based diesel additive (called EnviroxTM), burned at 850 °C to mimic its release after combustion in a diesel engine. We demonstrated significant DNA damage after in vitro exposure to the lowest tested concentration (1 µg·L−1) using the alkaline comet assay (ACA). We also showed a significant increase in oxidative stress in human sperm after in vitro exposure to 1 µg·L−1 aged CeO2 NMs evaluated by the H2DCF-DA probe. Electron microscopy showed no internalization of aged CeO2 NMs in human sperm but an affinity for the head plasma membrane. The results obtained in this study provide some insight on the complex cellular mechanisms by which aged CeO2 NMs could exert in vitro biological effects on human spermatozoa and generate ROS.
Keywords: nanoparticles, DNA damage, Oxidative stress, reproductive toxicity, combustion, ageing, NMs life cycle
1. Introduction
Nanoparticles (NPs) and nanomaterials (NMs), particularly metal oxide NMs, are increasingly used in many fields of everyday life, e.g., food packaging, cosmetics, textiles, electronics, and even biomedicine. Extensive usage of NMs in various areas has raised human health concerns, mostly in terms of occupational exposure [1,2]. Considering the life cycle of nano-enabled products (from the production and formulation stage to their usage and end of life), the occupational exposure of workers to NMs [3] has been more studied than others viz., consumer and environmental exposures. The reason is the presence of non-nano-specific regulations, which put worker protection at the forefront with efficient individual protection equipment [4]. Consequently, major knowledge gaps remain in the risk assessment of NMs, especially in the post-production stages of their life cycle [5], e.g., people/consumers are directly or indirectly exposed during use or during waste treatment.
Among all, CeO2 NMs have been increasingly used in Europe and elsewhere as fuel-borne catalysts in diesel engines [6,7] as the EnviroxTM from Energenics Europe Ltd. The addition of CeO2 NMs in diesel has been reported to increase the fuel combustion efficiency [8] and decrease the emission of CO2, CO, the total particulate mass, formaldehyde, acetaldehyde, acrolein, and several polycyclic aromatic hydrocarbons [9] during combustion. However, it has also been shown to increase the emission of ultrafine particles, NOx, and benzo[a]pyrene as well as the amount of Ce released in natural environments (air, water, soil) near roads [10,11]. In the UK, near an urban road where EnviroxTM is known to be used, an increase in ambient Ce-based NMs was observed with a concentration of ∼0.3 ng·m−3 and aerodynamic diameters peaking at 150 nm [12]. To date, there are large uncertainties regarding the acceptable level of Ce-based NMs in the atmosphere because most toxicity studies have been done with pristine CeO2 NMs that are not representative of the emission in diesel exhaust. Indeed, once released in the atmosphere after combustion in a diesel engine, Ce is in the form of CeO2 NMs with different physical-chemical properties compared to pristine CeO2 NMs (in terms of size, surface properties, mineralogy, aggregation state, solubility) [4], which can affect their fate, bioavailability [13,14] and potential toxicity [2,15]. Therefore, investigating the potential adverse effects of such combusted CeO2 NMs on human health represents an important step in the safety assessment required from Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) and from the Organization for Economic Cooperation and Development (OECD’s guidelines 2011) [16,17].
Atmospheric pollution is known to affect numerous physiological functions, including human reproduction and fertility, and it is reported to be related to lower semen quality [17,18,19,20,21,22], reduced fertility and spontaneous fertility rate [18,19,20], and reduced success rates of in vitro fertilisation (IVF) in humans [21,22]. Regarding the reprotoxicity of NMs, controversial reports have been published in recent years with particular attention to the effects on male gametes [23]. Few in vivo and in vitro studies have estimated the potential harmful impacts of CeO2 NMs on reproductive organs and germ cells. Pristine CeO2 NMs (5–40 nm) have been shown to cross the blood–testis barrier and accumulate in the testis (<0.2% of the inhaled dose) of rats following 28 days of inhalation in vivo (total estimated inhaled dose 0.83–4.24 mg/rat CeO2 NMs) [24]. Accumulation of Ce in the testes of mice was also observed after 32 days of in vivo oral administration of pristine CeO2 NMs (27.62 ± 3.01 nm) at the highest doses of 20 or 40 mg/kg body weight [25]. This accumulation caused a decrease in daily sperm production, lower motility, and sperm DNA damage [25]. Our team recently demonstrated the in vitro genotoxicity of pristine CeO2 NMs (7 nm) on human sperm cells and mouse gametes after exposure to 10 µg·L−1 [26,27]. Significant impairments in fertilisation rates were observed in mice [26], and a significant increase in DNA damage in human sperm [27]. The mechanisms of DNA damage were indirectly attributed to oxidative stress via the adjunction of an antioxidant (L-ergothineine) in the exposure medium [27].
Most of the previous studies were conducted with pristine CeO2 NMs (i.e., at the production stage of the life cycle), which does not reflect a realistic exposure route of men and women to Ce-based NMs that are likely released in diesel exhaust. However, the WHO’s International Agency for Research on Cancer classified diesel engine exhaust as carcinogenic to humans [28], and the OECD classified CeO2 NMs as part of a priority list of NMs whose potential toxicity require urgent evaluation [16]. Hence, the potential reproductive toxicity [29] of combusted CeO2 NMs must be evaluated.
This study was designed to evaluate the potential genotoxicity induced by in vitro exposure of human and rat sperm cells to low concentrations of combusted commercialised CeO2 NM-based diesel additives (EnviroxTM). Combining biological assays with physico-chemical characterisation, we addressed two questions: (i) Do combusted CeO2 NMs from a diesel additive induce genotoxic effects towards human and rat sperm cells following in vitro exposure to low concentrations? (ii) Were the mechanisms of genotoxicity and the interactions with sperm cells different from pristine CeO2 NMs?
2. Material and Methods
2.1. Physical-Chemical Characterisation
2.1.1. Ageing of the Diesel Fuel Additive
CeO2 NMs were recovered from EnviroxTM, Energenics Europe Ltd., Oxfordshire, UK, which is a fuel-borne catalyst scientifically and commercially proven CeO2 NM-based diesel additive supplied by Energenics Europe Ltd. EnviroxTM was combusted following the protocol published in [4]. Briefly, we ultracentrifuged EnviroxTM suspensions at 396,750× g and 20 °C for 1 h and removed the supernatant to recover the pellet containing CeO2 NMs. The pellets were freeze-dried (Heto PowerDry LL3000, Thermo Fisher Scientific, Strasbourg, France) for 5 days. The dried samples were crushed, and a total amount of 1.2 g was introduced in a furnace at 850 °C (i.e., at the average combustion temperature of diesel engines) [30] for 20 min. A stock suspension of the combusted EnviroxTM (called aged CeO2 NMs) was prepared in Milli-Q water at 10.15 g·L−1 of CeO2. The combusted EnviroxTM contained CeO2 NMs with an average TEM size of 19 ± 10 nm, a larger polydispersity than the uncombusted ones, and a polyhedral shape [4]. We used X-ray diffraction (XRD) (X′Pert-Pro diffractometer, PANalytical) and transmission electron microscopy (TEM) (FEI 2Tecnaiï G2) to confirm the size, shape, and mineralogy of the produced NMs (see Supplementary Materials, Figure S1).
2.1.2. Aged CeO2 NM Dissolution in FertiCult® Medium
We assessed the dissolution of aged CeO2 NMs in FertiCult® medium (JCD Laboratories, Lyon, France) for the in vitro culture of mammalian gametes by inductively coupled plasma mass spectrometry (ICP-MS) (NexION 300X, Perkin Elmer®). We incubated aged CeO2 NMs at room temperature (RT) in the culture medium for 2 and 5 h at three concentrations (10, 1000, and 100,000 µg·L−1). After incubation, the suspensions were ultra-filtered at 3 KDa (Amicon Ultra-15, Merck, France) at 3000× g for 1 h and by ICP-MS; triplicates were performed for each concentration.
2.2. Gamete Collection
Rat sperm cell collection. After sacrifice, we collected and cut the epididymis to enable the exit of sperm in HTF-BSA culture medium (Human Tubal Fluid, Millipore®, France, with 0.4% BSA: Bovine Serum Albumin, Sigma-Aldrich®, Lyon, France) for 1 h at 37 °C and CO2 5%.
Human sperm collection. We used frozen human sperm from healthy fertile donors. After thawing, we aliquoted the preparation and centrifuged it for 10 min at 420 g. The supernatants were discarded, and the pellets were exposed to various exposure conditions.
2.3. Ethical Authorization
Ethical authorization for animal sampling of gametes was obtained from the National Ethics Committee on Animal Experimentation (2018061110211950-V2 #15447). We used Sprague Dawley rats, Oncins France Strain A (623OFA), which were purchased from Charles River Laboratories, France. Sexually mature male rats of 60 days old were housed with free access to food and water until sacrifice.
Human sperm cells were purchased from GERMETHEQUE biobank, which obtained informed consent from each donor for inclusion of samples in the biobank and for their use in research experiments regarding human fertility in accordance with the 1975 Helsinki Declaration on human experimentation.
For oxidative stress analysis, the principle of Replacement of the 3R rule (reduction, refinement, and replacement) was applied and human sperm only was used.
2.4. Sperm Exposure
We exposed human and rat sperm to four different concentrations of aged CeO2 NMs (1, 10, 100, and 1000 µg·L−1) for 1 h at 37 °C and 5% CO2. FertiCult® medium was used as a negative control and 110 µmol·L−1 H2O2 as a positive control. The H2O2 concentration was chosen based on previous studies [26,27,31]. At least three different experiments were performed. After exposure, we recovered all motile sperm cells by swim-up [32], and before comet assay analysis, we measured sperm viability by eosin-nigrosine staining according to the World Health Organisation (WHO, 1999, Appendix IV.2) technique (100 cells were evaluated per condition).
2.5. DNA Damage Evaluation by Comet Assay
We performed the alkaline comet assay according to the procedure described by Singh [33] and adapted by Baumgartner [34], which has already been described in previous publications by our team [26,27,31]. DNA damage was quantified by the percentage of DNA in the tail of 100 randomly selected sperm cells from each triplicate slide per condition (at least 300 raw values analysed per experiment, at least 900 in total per condition).
Statistical Analysis. The data presented the medians of % Tail DNA values, with 1st and 3rd quartiles. We performed a linear mixed model analysis with “condition” (exposure condition) as a fixed effect and “cells” (sperm cells) within the replicate slide as a random effect using linear mixed effects regression (lmer) function of R software, version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria) to compare DNA damage among the various conditions. Pairwise differences of least-squares mean for all conditions were post-hoc assessed. Statistical significance was set at p < 0.05.
2.6. Oxidative Stress Analysis on Human Sperm
We investigated the effect on human sperm of aged CeO2 NMs in vitro exposure on the generation of reactive oxygen species (ROS), at the exposure concentration that had induced the higher DNA damage, i.e., 1 µg·L−1. At least three different experiments were performed. We used a 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) probe [35]. The exposure protocol was readapted from Aitken et al. (2013) and Gallo et al. (2018) [36,37]. After thawing and dilution in FertiCult® culture medium, we centrifuged human sperm for 10 min at 420× g, discarded the supernatant and added 10 µmol·L−1 H2DCF-DA [36] at 37 °C and 5% CO2 for 45 min to enable internalization of the permeable probe. Then, the sperm cells were washed and exposed to 1 µg·L−1 CeO2 NMs for 1 h at 37 °C and 5% CO2. We used H2O2 110 µmol·L−1 as a positive control and FertiCult® medium as a negative control. After 1 h, we recovered the motile sperm by swim-up selection. For vitality staining, we exposed the sperm to 4′,6-diamidino-2-phenylindole (DAPI) at 0.5 µg·mL−1 immediately prior to analysis by flow cytometry (CytoFLEX, Beckman Coulter, IN, USA). The results are only based on a live sperm population and expressed as the percentage of DCF-positive cells (expressing the fluorescence) in 100,000 events per condition in each replicate experiment (300,000 live cells in total per condition).
2.7. Imaging of Human Sperm Cells after In Vitro Exposure
Human sperm were exposed in vitro to 1 and 100 µg·L−1 of aged CeO2 NMs and selected by swim-up after 1 h. Non-exposed sperm cells were used as the control. Recovered motile sperm cells were washed with 0.1 M phosphate buffer, then fixed with glutaraldehyde 2.5% in 0.1 M phosphate buffer during 30 min at RT, and finally rinsed 3 times with 0.1 phosphate buffer. Samples were post-fixed with 2% osmium tetroxyde in 0.1M phosphate buffer during 30 min and washed 3 times with 0.1 M phosphate buffer. Progressive dehydration with 50% to 100% Ethanol bath was performed before embedding in Epon 812 (epoxy resin) from 33% to 100% EPON. Ultrathin sections (60 nm) were obtained using Ultracut-E ultramicrotome (Reichert-Jung, Southbridge, MA, USA) and contrast was performed using Uranyl acetate 5% for 12 min and dried at room temperature. Pictures were obtained using a JEM 1400 transmission electron microscope (JEOL, Tokyo, Japan) at 80 kV with a Megaview III camera under iTEM Five software (SIS Imaging, Münster, Germany). This procedure was described in [27].
3. Results
3.1. No Detectable Dissolution of Aged CeO2 NMs in Abiotic Conditions
The dissolution (<3 kDa) of CeO2 NMs in abiotic FertiCult® medium (pH 7.2–7.5) was studied after 2 and 5 h of incubation at 10, 1000, and 100,000 µg·L−1. Regardless of the concentrations tested, no dissolution of the CeO2 NMs could be measured by ICP-MS in abiotic conditions. Below 3 kDa, the concentrations of dissolved Ce species were under the limits of detection (i.e., <0.002 µg·L−1).
3.2. Higher DNA Damage Detected at the Lowest Concentration Exposure
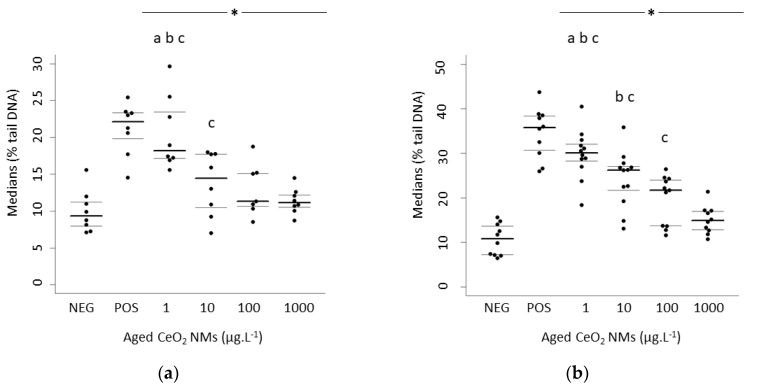
Rat and human sperm cells were exposed in vitro for one hour to 0, 1, 10, 100, or 1000 µg·L−1 of aged CeO2 NMs. After exposure, all viability rates exceeded the normality threshold as stated by the WHO criteria [38]. The results are presented as the distribution of median values of % tail DNA with 1st and 3rd quartiles, obtained from 3 independent experiments. p < 0.05, for the differences compared versus *: negative control (NEG), a: vs. 10 µg·L−1 CeO2 NMs, b: vs. 100 µg·L−1 CeO2 NMs, c: vs. 1000 µg·L−1 CeO2 NMs (Figure 1a,b).
Figure 1.
Evaluation of DNA damage using the Comet Assay following in vitro exposure of rat (a) and human sperm (b) to aged CeO2 NMs. Tested concentrations: 0, 1, 10, 100, and 1000 µg·L−1 of aged CeO2 NMs, POS (110 µM H2O2, positive control). p < 0.05, for differences compared versus *: negative control (NEG); a: vs. 10 µg·L−1 CeO2 NMs; b: vs. 100 µg·L−1 CeO2 NMs; c: vs. 1000 µg·L−1 CeO2 NMs. In each condition, the main central line corresponds to the median of the points and the two other lines correspond to 1st and 3rd quartiles.
In rat- and human-exposed sperm cells, all exposure concentrations induced significantly higher DNA damage than the negative control (p < 0.05) (Figure 1a,b). Furthermore, a significant increase in DNA damage was observed at the lowest tested concentration (1 µg·L−1) (medians of % tail DNA in rats and humans of 18.20 and 30.10, respectively) compared to the exposure to 10, 100, and 1000 µg·L−1 aged CeO2 NMs; (p < 0.001). We also observed a significant difference between 10 and 1000 µg·L−1 aged CeO2 NMs in rat sperm (p < 0.05) and a significant difference among the three highest tested concentrations in human sperm (p < 0.05). The variability of biological data in the 3 independent experiments is presented in Table 1.
Table 1.
Biological variability of the data. Median values of % Tail DNA of each condition of three experiments, with 1st and 3rd quartiles.
| Rat Sperm Cells | MEDIAN Values | 1st Quartile | 3rd Quartile | Human Sperm Cells | MEDIAN values | 1st Quartile | 3rd Quartile |
|---|---|---|---|---|---|---|---|
| Negative control | 9.34 | 7.94 | 11.24 | Negative control | 10.8 | 7.26 | 13.7 |
| 1 µg·L−1 CeO2 | 18.2 | 17.17 | 23.47 | 1 µg·L−1 CeO2 | 30.1 | 28.28 | 32.06 |
| 10 µg·L−1 CeO2 | 14.46 | 10.48 | 17.72 | 10 µg·L−1 CeO2 | 26.24 | 21.69 | 27.09 |
| 100 µg·L−1 CeO2 | 11.31 | 10.64 | 15.13 | 100 µg·L−1 CeO2 | 21.74 | 13.72 | 23.99 |
| 1000 µg·L−1 CeO2 | 11.15 | 10.53 | 12.19 | 1000 µg·L−1 CeO2 | 14.91 | 12.88 | 17.02 |
3.3. Oxidative Stress Detected in Human Sperm
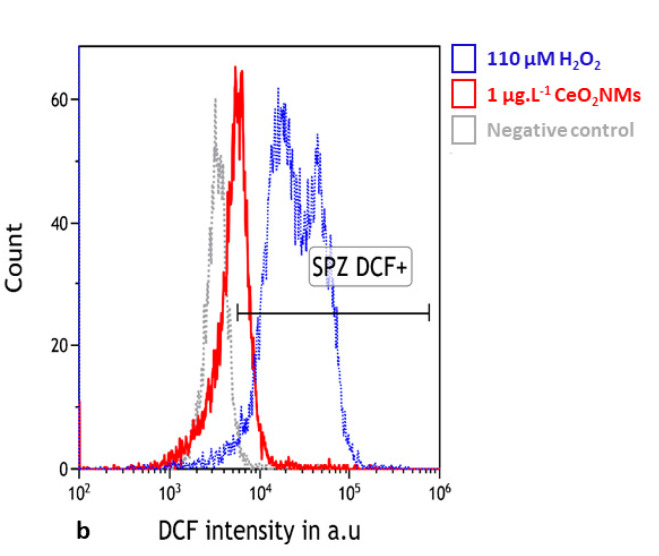
After 1 h of in vitro exposure to 1 µg·L−1 aged CeO2 NMs, the percentage of DCF-positive live sperm (mean ± SEM= 13.1 ± 3.9) significantly increased compared to the negative control (5.21 ± 2.9) (p = 0.047) (Figure 2).
Figure 2.
Detection of reactive oxygen species (ROS) using the H2DCF-DA probe. Human sperm cells were exposed in vitro for one hour to 1 µg·L−1 aged CeO2 NMs. Intracellular reactive oxygen species were evaluated by the dichlorodihydrofluorescein (DCF) intensity. The results are representative of 3 independent experiments. p < 0.05, for differences compared versus negative control. SPZ: spermatozoa. a.u: arbitrary unit.
3.4. Aged CeO2 NMs Detected by TEM on the Plasma Membrane of Human Sperm
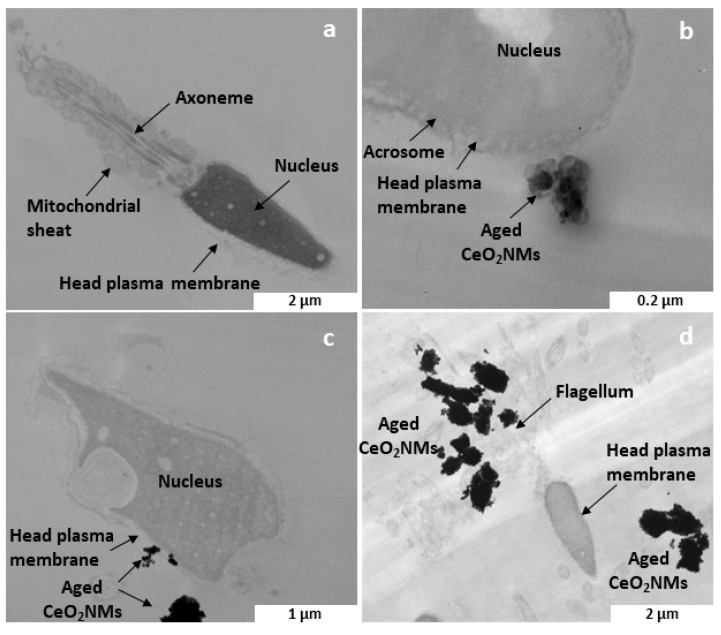
In human sperm cells, TEM detected aged CeO2 NM aggregates near the sperm plasma membrane after the exposure to 1 µg·L−1 CeO2 NMs (Figure 3b,c). After the exposure to 100 µg·L−1, the NMs appeared even more aggregated and not in close interaction with the cells (Figure 3d). No CeO2 NM internalization was observed by TEM at any exposure concentration.
Figure 3.
TEM analysis of aged CeO2 NMs in human sperm after in vitro exposure. (a) negative control; (b,c) exposed to 1 µg·L−1 CeO2 NMs; (d): exposed to 100 µg·L−1 CeO2 NMs. The white rectangles correspond to the scale bar.
4. Discussion
4.1. Exposure to Low Doses of Aged CeO2 NMs Induced Higher DNA Damage
One major drawback of the currently used studies and models to evaluate the toxicity of NMs for humans is the lack of relevance of the exposure scenario (i.e., relevant concentration of NMs, relevant speciation of the tested NMs) [39]. Indeed, most in vivo studies are based on theoretical data regarding the potential exposure of humans to CeO2 NMs. For example, Modrzynska (2018) exposed mice to a single dose of 162 μg of CeO2 or TiO2 NMs in 50 μL of 2% serum in nano-pure water [40]. This single dose corresponds to pulmonary deposition during 138-h working days at the Danish occupational exposure limit of 10 mg/m3 for TiO2 assuming 9% alveolar deposition [41,42]. Li (2016) exposed rats to CeO2 NMs in a nose-only exposure system for a single 4-h exposure (27–39.8 μg/m3). They used a scanning mobility particle sizer to estimate the average mass concentration and compared it with the physical sampling measurements based on the filter packs [43].
Here, we highlighted the importance of the dose and more particularly of the low dose. We expected a moderate release of CeO2 NMs in the air from a diesel engine and a spread into the gonads, as shown by Qin et al. (2019) and Préaubert et al. (2015) [25,26]. The lowest concentration of aged CeO2 NMs (1 µg·L−1) was found to induce the highest DNA damage in both human and rat sperm cells (Figure 1a,b). These results are consistent with our previous study, which showed inverse dose-response DNA damage after the in vitro exposure of human sperm to pristine CeO2 NMs [27]. Few hypotheses could be formulated to explain the highest genotoxicity at the lowest exposure concentration. One refers to different NMs/cells interactions because of dose-dependent aggregation states. Herein, it was not technically possible (below the detection limit) to measure the size of NMs aggregates at 1 µg·L−1 and 1000 µg·L−1 of CeO2 NMs in the FertiCult® medium. However, the strong aggregation due to combustion already observed in reference [4] is also expected at the pH and ionic strength of extracellular media. Based on the dose-dependent probability of contact between NMs, smaller aggregates should be expected at the lowest concentration (1 µg·L−1 CeO2). In that case, smaller aggregates would be more prone to interact with the cells and enhance their biotransformation, biological, and toxicological effects [44]. It is known that nanoparticle genotoxicity and cytotoxicity are controversial, especially because these interactions are species-specific, often tissue-specific, and related to physico-chemical features of exposure medium [45,46]. Moreover, nanoparticles show a strong tendency to form agglomerates in solution due to their high surface area. It is a general opinion that the degree and type of agglomerates formed may influence the toxicity of NPs [47,48].
4.2. DNA Damage in Human Sperm Was Associated with Oxidative Stress
A generally accepted paradigm is that the toxicity of NMs arises primarily because they can generate reactive oxygen species (ROS) and oxidative damage [44]. Oxidative stress is a major cause of DNA damage in mammalian spermatozoa [49], since it can affect the membrane integrity and motility [50,51], and it is also associated with failures of fertilisation, abnormal embryonic development, and premature pregnancy loss [52].
Understanding the mechanism of action of CeO2 NMs is a major challenge since they are known to exhibit pro- and antioxidant properties. For example, Das et al. (2007) [53] and Niu et al. (2007) [54] showed that CeO2 NMs (3–5 and 7 nm, respectively) could reduce oxidative stress as free radical scavengers, whereas Auffan et al. (2009) [55] highlighted the ability of CeO2 NMs (7 nm) to damage fibroblast DNA at very low doses (6–1.2 × 106 µg·L−1). The determining factors are the stoichiometry, oxido-reduction state of Ce, pH of the medium, presence of H2O2, etc. [56], but it also depends on the cell types, organisms [57], size and speciation of the NMs [58].
Herein, we demonstrated in human sperm that 1 h of in vitro exposure to 1 µg·L−1 aged CeO2 NMs induced a significant increase in ROS generation. The H2DCF-DA probe becomes fluorescent on oxidation and is purported to directly monitor reactive oxygen and/or nitrogen species (ROS/RNS) [59]. ONOO- and the hydroxyl radical also directly oxidise this probe and significantly contribute to the positive signals observed in defective human spermatozoa [60,61]. Our results confirm previous studies, where we showed that DNA damage was induced in human sperm by exposure to 10 µg·L−1 of pristine CeO2 NMs was significantly decreased by the addition of an antioxidant (L-ergothioneine) in the exposure medium [27]. L-ergothioneine is known to scavenge hydroxyl radicals, hypochlorous acid, and peroxynitrite [62], which can directly oxidise the H2DCF-DA probes [60,61]. We hypothesize that oxidative stress can be one of the mechanisms responsible for the DNA damage detected in sperm cells.
At 1 µg·L−1, aged CeO2 NMs were found in close contact with the plasma membrane of the head of the spermatozoa. This interaction with the sperm head associated with the highest sensitivity of the H2DCF-DA probe when oxidants are generated near the plasma membrane or in the cytoplasm [36] can help us elucidate the mechanisms of oxidative stress generated by aged CeO2 NMs. Indeed, the head of the spermatozoa expresses various ion channels (e.g., Ca2+ and K+) [63,64], which offer entry paths for metallic toxicants such as Zn2+ and Pb2+ into a mature spermatozoa [63,65,66]. More interestingly, some trivalent ions such as La3+ and Ce3+ act as T-type calcium channel antagonists and competitively bind and block the Ca2+ binding sites [67,68], which are required for the sperm head mannose receptors [65]. Then, mannose receptor expression is considered a biomarker for the effects of transition and heavy metals and organic toxicants on sperm fertility potential [66].
Here, we did not observe any abiotic dissolution of Ce in the Ferticult® medium. However, reductive dissolution of nanocrystalline Ce(IV)O2 into Ce(III) is pH-dependent at pH < 7 [69,70,71] and it is possible that a release of Ce(III) with a pro-oxidant activity could occur in the vicinity of the cells due to the metabolic activity [69,70]. Indeed, in sperm cells, voltage-gated proton channels Hv1 (i.e., the main H(+) extrusion pathway) activated by alkaline extracellular environment induce an alkalinisation of intracellular pH necessary for the functional activation (capacitation) of sperm, and consequently a decrease in extracellular pH [72]. Such an extracellular pH decrease could locally enhance the dissolution of CeO2 NMs in the vicinity of the cells. More information on the biodistribution and biotransformation of CeO2 at the scale of the cell membrane is required to validate the hypothesis that the observed oxidative stress can be attributed to the possible biotransformation of CeO2 at the surface of the plasma membrane.
4.3. Lifecycle Stage-Dependent (Geno)Toxicity of CeO2 NMs
To date, few studies have explored the DNA damage and oxidative stress induced by pristine CeO2 NMs (mostly monodisperse and homogeneously coated), which are not representative of CeO2 NMs that are likely released during use. One strength of this study is the use of combusted EnviroxTM to mimic real exposure to CeO2 NMs, which are potentially released from diesel vehicles equipped with an active nano-catalyst for soot abatement. With an over-combustion in a single-cylinder engine with mechanical fuel injection [13] or in an oven at a temperature close to that of diesel engines [4], the physico-chemical properties of CeO2 NMs evolve compared to those initially incorporated in the diesel additive: the size of the CeO2 crystallites significantly increased (i.e., the decrease in specific surface area from 113 ± 17 to 63 ± 35 m2·g−1) without detectable Ce(III) in the structure, and no organic compounds remained at the surface. This transformation upon combustion highlights that at each stage of the nano-enabled product life cycle (formulation, usage/combustion, and end of life), the mechanisms and kinetics of interactions between the released CeO2 NMs and the aqueous media (e.g., water, soil, biological media) and exposed organisms will differ [5,73]. Hence, the life cycle stages of nano-enabled products when studying their toxicity is critical [62,74]. This is particularly the case when studying their genotoxicology [75] towards gametes since fertility may be altered, which subsequently affects the reproduction rate and health of the offspring [26,76].
Based on previously published data and the current study, we compared the impacts towards gametes of pristine CeO2 NMs (production stage of the life cycle) and combusted diesel additives (usage stage of the lifecycle). At both lifecycle stages, a higher genotoxicity attributed to oxidative stress was observed at the lowest doses towards human sperm cells [26,27]. For pristine and combusted NMs, an inverse dose–effect relationship was attributed to different aggregation states (size and density). At low doses, the probability of contact between two particles (homo-aggregation) decreases, and CeO2 NMs are likely more dispersed, which increases the contact surface between NMs and cells [27]. However, for pristine NMs only, the significant genotoxicity at low doses was also attributed to their chemical instability because of their larger specific surface area and an organic coating at the surface [4].
5. Conclusions
We demonstrated for the first time the DNA damage induced in human and rat sperm cells after exposure to low concentrations of CeO2 NMs, which are similar to the ones released by combustion in a diesel engine. In vitro exposure to the lowest concentration of combusted CeO2 NM induced oxidative stress in human sperm cells after the interaction with the plasma membrane. While important from a mechanistic standpoint, this study remains limited by its in vitro nature. However, in vitro exposure is a relevant design since spermatozoa can encounter nanoparticles in the male gonads or female genital tract. In vivo studies after a low-dose exposure to aged NMs will help us more closely approach realistic exposure conditions. Then, we will be able to decipher the transfer of CeO2 NMs at different stages of the lifecycle to various organs in animals, their permeation of biological membranes, accumulation in reproductive organs, and their impact on embryonic development, as conducted with pristine NMs [67,77,78,79,80]. To go further in the relevance of the exposure scenario, the complexity of the exhaust emissions [81] should be considered when assessing their genotoxicity. For instance, a considerable amount of Polycyclic Aromatic Hydrocarbons (PAHs) and their alkylated derivatives are emitted by diesel engines [82]. Some of them are well known for their carcinogenic and reprotoxic potential, such as benzo[a]pyrene (BaP) [83]. An even more relevant exposure scenario can assess the potential hazard due to co-exposure to combusted CeO2 NMs associated with other organic compounds generated during diesel combustion.
Acknowledgments
This work is a contribution to the Labex Serenade (No. ANR-11-LABX-0064) funded by the “Investissements d’Avenir” French Government program of the French National Research Agency (ANR) through the A MIDEX project (No. ANR-11-IDEX-0001-02). This work is also a contribution to the OSU-Institut Pytheéas. The authors acknowledge the CNRS for the funding of the IRP iNOVE.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-4991/10/12/2327/s1. Figure S1: X-ray diffractogram and transmission electron microscopy image of the 850 °C aged CeO2 NMs.
Author Contributions
Conceptualization, M.A., J.R. and J.P.; Data curation, M.C.; Formal analysis, M.C. and N.R.; Funding acquisition, M.A., J.R. and J.P.; Investigation, M.C.; Methodology, M.C., S.R. and V.T.; Project administration, J.R. and J.P.; Supervision, M.A., J.R. and J.P.; Validation, M.A., J.R. and J.P.; Visualization, J.P.; Writing–original draft, M.C.; Writing–review & editing, M.C., M.A., J.R. and J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No713750. Additionally, it has been performed with the financial supports of the Regional Council of Provence-Alpes-Côte d’Azur and A*MIDEX (n° ANR- 11-IDEX-0001-02), funded by the Investissements d’Avenir project funded by the French Government and managed by the French National Research Agency (ANR). The project has also received funding from Fédération de Recherche ECCOREV n° 3098.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lee J., Jeong J.-S., Kim S.Y., Lee S.-J., Shin Y.-J., Im W.-J., Kim S.-H., Park K., Jeong E.J., Nam S.-Y., et al. Safety assessment of cerium oxide nanoparticles: Combined repeated-dose toxicity with reproductive/developmental toxicity screening and biodistribution in rats. Nanotoxicology. 2020:1–15. doi: 10.1080/17435390.2020.1813825. [DOI] [PubMed] [Google Scholar]
- 2.Oberdörster G., Oberdörster E., Oberdörster J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ. Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graczyk H., Riediker M. Occupational exposure to inhaled nanoparticles: Are young workers being left in the dust? J. Occup. Health. 2019;61:333–338. doi: 10.1002/1348-9585.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auffan M., Tella M., Liu W., Pariat A., Cabié M., Borschneck D., Angeletti B., Landrot G., Mouneyrac C., Giambérini L., et al. Structural and physical–chemical behavior of a CeO2 nanoparticle based diesel additive during combustion and environmental release. Environ. Sci. Nano. 2017;4:1974–1980. doi: 10.1039/C7EN00494J. [DOI] [Google Scholar]
- 5.Masion A., Auffan M., Rose J. Monitoring the Environmental Aging of Nanomaterials: An Opportunity for Mesocosm Testing? Materials. 2019;12:2447. doi: 10.3390/ma12152447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaiser B.K., Fernandes T.F., Jepson M., Lead J.R., Tyler C.R., Stone V. Assessing exposure, uptake and toxicity of silver and cerium dioxide nanoparticles from contaminated environments. Environ. Health. 2009;8:S2. doi: 10.1186/1476-069X-8-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park B., Martin P., Harris C., Guest R., Whittingham A., Jenkinson P., Handley J. Initial in vitro screening approach to investigate the potential health and environmental hazards of EnviroxTM—A nanoparticulate cerium oxide diesel fuel additive. Part. Fibre Toxicol. 2007;4:12. doi: 10.1186/1743-8977-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung H., Kittelson D.B. Measurement of Electrical Charge on Diesel Particles. Aerosol Sci. Technol. 2005;39:1129–1135. doi: 10.1080/02786820500430357. [DOI] [Google Scholar]
- 9.Zhang J., Nazarenko Y., Zhang L., Calderon L., Lee K.-B., Garfunkel E., Schwander S., Tetley T.D., Chung K.F., Porter A.E., et al. Impacts of a Nanosized Ceria Additive on Diesel Engine Emissions of Particulate and Gaseous Pollutants. Environ. Sci. Technol. 2013;47:13077–13085. doi: 10.1021/es402140u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park B., Donaldson K., Duffin R., Tran L., Kelly F., Mudway I., Morin J.-P., Guest R., Jenkinson P., Samaras Z., et al. Hazard and Risk Assessment of a Nanoparticulate Cerium Oxide-Based Diesel Fuel Additive—A Case Study. Inhal. Toxicol. 2008;20:547–566. doi: 10.1080/08958370801915309. [DOI] [PubMed] [Google Scholar]
- 11.Johnson A.C., Park B. Predicting contamination by the fuel additive cerium oxide engineered nanoparticles within the United Kingdom and the associated risks. Environ. Toxicol. Chem. 2012;31:2582–2587. doi: 10.1002/etc.1983. [DOI] [PubMed] [Google Scholar]
- 12.Gantt B., Hoque S., Willis R.D., Fahey K.M., Delgado-Saborit J.M., Harrison R.M., Erdakos G.B., Bhave P.V., Zhang K.M., Kovalcik K., et al. Near-Road Modeling and Measurement of Cerium-Containing Particles Generated by Nanoparticle Diesel Fuel Additive Use. Environ. Sci. Technol. 2014;48:10607–10613. doi: 10.1021/es502169p. [DOI] [PubMed] [Google Scholar]
- 13.Batley G.E., Halliburton B., Kirby J.K., Doolette C.L., Navarro D., McLaughlin M.J., Veitch C. Characterization and ecological risk assessment of nanoparticulate CeO2 as a diesel fuel catalyst: Characterization and risk of nano-CeO2 as a diesel catalyst. Environ. Toxicol. Chem. 2013;32:1896–1905. doi: 10.1002/etc.2246. [DOI] [PubMed] [Google Scholar]
- 14.Dale J.G., Cox S.S., Vance M.E., Marr L.C., Hochella M.F. Transformation of Cerium Oxide Nanoparticles from a Diesel Fuel Additive during Combustion in a Diesel Engine. Environ. Sci. Technol. 2017;51:1973–1980. doi: 10.1021/acs.est.6b03173. [DOI] [PubMed] [Google Scholar]
- 15.Cassee F.R., van Balen E.C., Singh C., Green D., Muijser H., Weinstein J., Dreher K. Exposure, Health and Ecological Effects Review of Engineered Nanoscale Cerium and Cerium Oxide Associated with its Use as a Fuel Additive. Crit. Rev. Toxicol. 2011;41:213–229. doi: 10.3109/10408444.2010.529105. [DOI] [PubMed] [Google Scholar]
- 16.OECD’s Guidelines OECD’s Meeting on Safety Testing of Manufactured Nanomaterials and Test Guidelines . [(accessed on 15 May 2020)];2011 Mar; Available online: http://www.oecd.org/science/nanosafety/48291037.pdf.
- 17.Haase A., Luch A. Genotoxicity of nanomaterials in vitro: Treasure or trash? Arch. Toxicol. 2016;90:2827–2830. doi: 10.1007/s00204-016-1825-5. [DOI] [PubMed] [Google Scholar]
- 18.Dejmek J., Jelínek R., Solansky’ I., Benes I., Srám R.J. Fecundability and parental exposure to ambient sulfur dioxide. Environ. Health Perspect. 2000;108:647–654. doi: 10.1289/ehp.00108647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nieuwenhuijsen M.J., Basagaña X., Dadvand P., Martinez D., Cirach M., Beelen R., Jacquemin B. Air pollution and human fertility rates. Environ. Int. 2014;70:9–14. doi: 10.1016/j.envint.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Slama R., Bottagisi S., Solansky I., Lepeule J., Giorgis-Allemand L., Sram R. Short-Term Impact of Atmospheric Pollution on Fecundability. Epidemiology. 2013;24:871–879. doi: 10.1097/EDE.0b013e3182a702c5. [DOI] [PubMed] [Google Scholar]
- 21.Legro R.S., Sauer M.V., Mottla G.L., Richter K.S., Li X., Dodson W.C., Liao D. Effect of air quality on assisted human reproduction. Hum. Reprod. 2010;25:1317–1324. doi: 10.1093/humrep/deq021. [DOI] [PubMed] [Google Scholar]
- 22.Perin P.M., Maluf M., Czeresnia C.E., Nicolosi Foltran Januário D.A., Nascimento Saldiva P.H. Effects of exposure to high levels of particulate air pollution during the follicular phase of the conception cycle on pregnancy outcome in couples undergoing in vitro fertilization and embryo transfer. Fertil. Steril. 2010;93:301–303. doi: 10.1016/j.fertnstert.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 23.Falchi L., Khalil W.A., Hassan M., Marei W.F.A. Perspectives of nanotechnology in male fertility and sperm function. Int. J. Vet. Sci. Med. 2018;6:265–269. doi: 10.1016/j.ijvsm.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geraets L., Oomen A.G., Schroeter J.D., Coleman V.A., Cassee F.R. Tissue Distribution of Inhaled Micro- and Nano-sized Cerium Oxide Particles in Rats: Results From a 28-Day Exposure Study. Toxicol. Sci. 2012;127:463–473. doi: 10.1093/toxsci/kfs113. [DOI] [PubMed] [Google Scholar]
- 25.Qin F., Shen T., Li J., Qian J., Zhang J., Zhou G., Tong J. SF-1 mediates reproductive toxicity induced by Cerium oxide nanoparticles in male mice. J. Nanobiotechnol. 2019:17. doi: 10.1186/s12951-019-0474-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Préaubert L., Courbiere B., Achard V., Tassistro V., Greco F., Orsiere T., Bottero J.-Y., Rose J., Auffan M., Perrin J. Cerium dioxide nanoparticles affect in vitro fertilization in mice. Nanotoxicology. 2015;10:111–117. doi: 10.3109/17435390.2015.1030792. [DOI] [PubMed] [Google Scholar]
- 27.Préaubert L., Tassistro V., Auffan M., Sari-Minodier I., Rose J., Courbiere B., Perrin J. Very low concentration of cerium dioxide nanoparticles induce DNA damage, but no loss of vitality, in human spermatozoa. Toxicol. In Vitro. 2018;50:236–241. doi: 10.1016/j.tiv.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Benbrahim-Tallaa L., Baan R.A., Grosse Y., Lauby-Secretan B., El Ghissassi F., Bouvard V., Guha N., Loomis D., Straif K. Carcinogenicity of diesel-engine and gasoline-engine exhausts and some nitroarenes. Lancet Oncol. 2012;13:663–664. doi: 10.1016/S1470-2045(12)70280-2. [DOI] [PubMed] [Google Scholar]
- 29.OECD’s Guidelines, Report of the OECD Expert Meeting on the Physical Chemical Properties of Manufactured Nanomaterials and Test Guidelines 2014. [(accessed on 15 May 2020)]; Available online: http://www.oecd.org/env/ehs/nanosafety/publications-series-safety-manufactured-nanomaterials.htm.
- 30.Flynn P.F., Durrett R.P., Hunter G.L., zur Loye A.O., Akinyemi O.C., Dec J.E., Westbrook C.K. Diesel Combustion: An Integrated View Combining Laser Diagnostics, Chemical Kinetics, and Empirical Validation. SAE International; Warrendale, PA, USA: 1999. [Google Scholar]
- 31.Einaudi L., Courbiere B., Tassistro V., Prevot C., Sari-Minodier I., Orsiere T., Perrin J. In vivo exposure to benzo(a)pyrene induces significant DNA damage in mouse oocytes and cumulus cells. Hum. Reprod. 2014;29:548–554. doi: 10.1093/humrep/det439. [DOI] [PubMed] [Google Scholar]
- 32.Perrin J., Tassistro V., Mandon M., Grillo J.-M., Botta A., Sari-Minodier I. Tobacco consumption and benzo(a)pyrene-diol-epoxide-DNA adducts in spermatozoa: In smokers, swim-up procedure selects spermatozoa with decreased DNA damage. Fertil. Steril. 2011;95:2013–2017. doi: 10.1016/j.fertnstert.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 33.Singh N.P., McCoy M.T., Tice R.R., Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 34.Baumgartner A., Cemeli E., Anderson D. The comet assay in male reproductive toxicology. Cell Biol. Toxicol. 2009;25:81–98. doi: 10.1007/s10565-007-9041-y. [DOI] [PubMed] [Google Scholar]
- 35.Chen X., Zhong Z., Xu Z., Chen L., Wang Y. 2′,7′-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free Radic. Res. 2010;44:587–604. doi: 10.3109/10715761003709802. [DOI] [PubMed] [Google Scholar]
- 36.Aitken R.J., Smith T.B., Lord T., Kuczera L., Koppers A.J., Naumovski N., Connaughton H., Baker M.A., De Iuliis G.N. On methods for the detection of reactive oxygen species generation by human spermatozoa: Analysis of the cellular responses to catechol oestrogen, lipid aldehyde, menadione and arachidonic acid. Andrology. 2013;1:192–205. doi: 10.1111/j.2047-2927.2012.00056.x. [DOI] [PubMed] [Google Scholar]
- 37.Gallo A., Manfra L., Boni R., Rotini A., Migliore L., Tosti E. Cytotoxicity and genotoxicity of CuO nanoparticles in sea urchin spermatozoa through oxidative stress. Environ. Int. 2018;118:325–333. doi: 10.1016/j.envint.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 38.WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. World Health Organization; Geneva, Switzerland: 2010. [Google Scholar]
- 39.Kuhlbusch T.A.J. Nanomaterial exposures for worker, consumer and the general public. NanoImpact. 2018;10:11–25. doi: 10.1016/j.impact.2017.11.003. [DOI] [Google Scholar]
- 40.Modrzynska J., Berthing T., Ravn-Haren G., Kling K., Mortensen A., Rasmussen R.R., Larsen E.H., Saber A.T., Vogel U., Loeschner K. In vivo-induced size transformation of cerium oxide nanoparticles in both lung and liver does not affect long-term hepatic accumulation following pulmonary exposure. PLoS ONE. 2018;13:e0202477. doi: 10.1371/journal.pone.0202477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hougaard K.S., Jackson P., Jensen K.A., Sloth J.J., Löschner K., Larsen E.H., Birkedal R.K., Vibenholt A., Boisen A.-M.Z., Wallin H., et al. Effects of prenatal exposure to surface-coated nanosized titanium dioxide (UV-Titan). A study in mice. Part. Fibre Toxicol. 2010;7:16. doi: 10.1186/1743-8977-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacobsen N.R., Møller P., Jensen K.A., Vogel U., Ladefoged O., Loft S., Wallin H. Lung inflammation and genotoxicity following pulmonary exposure to nanoparticles in ApoE−/− mice. Part. Fibre Toxicol. 2009;6:2. doi: 10.1186/1743-8977-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li D., Morishita M., Wagner J.G., Fatouraie M., Wooldridge M., Eagle W.E., Barres J., Carlander U., Emond C., Jolliet O. In vivo biodistribution and physiologically based pharmacokinetic modeling of inhaled fresh and aged cerium oxide nanoparticles in rats. Part. Fibre Toxicol. 2016;13:45. doi: 10.1186/s12989-016-0156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu P.P., Xia Q., Hwang H.-M., Ray P.C., Yu H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014;22:64–75. doi: 10.1016/j.jfda.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rocco L., Santonastaso M., Mottola F., Costagliola D., Suero T., Pacifico S., Stingo V. Genotoxicity assessment of TiO2 nanoparticles in the teleost Danio rerio. Ecotoxicol. Environ. Saf. 2015;113:223–230. doi: 10.1016/j.ecoenv.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 46.Mottola F., Iovine C., Santonastaso M., Romeo M.L., Pacifico S., Cobellis L., Rocco L. NPs-TiO2 and Lincomycin Coexposure Induces DNA Damage in Cultured Human Amniotic Cells. Nanomaterials. 2019;9:1511. doi: 10.3390/nano9111511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohanraj V.J., Chen Y. Nanoparticles—A review. Trop. J. Pharm. Res. 2006;5:561–573. doi: 10.4314/tjpr.v5i1.14634. [DOI] [Google Scholar]
- 48.Sager T.M., Porter D.W., Robinson V.A., Lindsley W.G., Schwegler-Berry D.E., Castranova V. Improved method to disperse nanoparticles for in vitro and in vivo investigation of toxicity. Nanotoxicology. 2007;1:118–129. doi: 10.1080/17435390701381596. [DOI] [Google Scholar]
- 49.Aitken R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017;84:1039–1052. doi: 10.1002/mrd.22871. [DOI] [PubMed] [Google Scholar]
- 50.Agarwal A. Oxidative Stress and Male Infertility: From Research Bench to Clinical Practice. J. Androl. 2002;23:737–752. doi: 10.1002/j.1939-4640.2002.tb02324.x. [DOI] [PubMed] [Google Scholar]
- 51.Aitken R.J., Gordon E., Harkiss D., Twigg J.P., Milne P., Jennings Z., Irvine D.S. Relative Impact of Oxidative Stress on the Functional Competence and Genomic Integrity of Human Spermatozoa. Biol. Reprod. 1998;59:1037–1046. doi: 10.1095/biolreprod59.5.1037. [DOI] [PubMed] [Google Scholar]
- 52.Aitken R.J., Curry B.J. Redox regulation of human sperm function: From the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid. Redox Signal. 2011;14:367–381. doi: 10.1089/ars.2010.3186. [DOI] [PubMed] [Google Scholar]
- 53.Das M., Patil S., Bhargava N., Kang J.-F., Riedel L.M., Seal S., Hickman J.J. Auto-catalytic Ceria Nanoparticles Offer Neuroprotection to Adult Rat Spinal Cord Neurons. Biomaterials. 2007;28:1918–1925. doi: 10.1016/j.biomaterials.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niu J., Azfer A., Rogers L.M., Wang X., Kolattukudy P.E. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc. Res. 2007;73:549–559. doi: 10.1016/j.cardiores.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Auffan M., Rose J., Wiesner M.R., Bottero J.-Y. Chemical stability of metallic nanoparticles: A parameter controlling their potential cellular toxicity in vitro. Environ. Pollut. 2009;157:1127–1133. doi: 10.1016/j.envpol.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 56.Sozarukova M.M., Proskurnina E.V., Baranchikov A.E., Ivanov V.K. CeO2 nanoparticles as free radical regulators in biological systems. Nanosyst. Phys. Chem. Math. 2020;11:324–332. doi: 10.17586/2220-8054-2020-11-3-324-332. [DOI] [Google Scholar]
- 57.Pulido-Reyes G., Rodea-Palomares I., Das S., Sakthivel T.S., Leganes F., Rosal R., Seal S., Fernández-Piñas F. Untangling the biological effects of cerium oxide nanoparticles: The role of surface valence states. Sci. Rep. 2015:5. doi: 10.1038/srep15613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hayes S.A., Yu P., O’Keefe T.J., O’Keefe M.J., Stoffer J.O. The Phase Stability of Cerium Species in Aqueous Systems. Part 1. E-pH Diagram for the Ce—HClO4—H2O System. ChemInform. 2002;34 doi: 10.1002/chin.200309018. [DOI] [Google Scholar]
- 59.Guo T., Cui L., Shen J., Wang R., Zhu W., Xu Y., Qian X. A dual-emission and large Stokes shift fluorescence probe for real-time discrimination of ROS/RNS and imaging in live cells. Chem. Commun. 2013;49:1862. doi: 10.1039/c3cc38471c. [DOI] [PubMed] [Google Scholar]
- 60.Myhre O., Andersen J.M., Aarnes H., Fonnum F. Evaluation of the probes 2′,7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem. Pharmacol. 2003;65:1575–1582. doi: 10.1016/S0006-2952(03)00083-2. [DOI] [PubMed] [Google Scholar]
- 61.Mahfouz R., Sharma R., Lackner J., Aziz N., Agarwal A. Evaluation of chemiluminescence and flow cytometry as tools in assessing production of hydrogen peroxide and superoxide anion in human spermatozoa. Fertil. Steril. 2009;92:819–827. doi: 10.1016/j.fertnstert.2008.05.087. [DOI] [PubMed] [Google Scholar]
- 62.Tang R.M.Y., Cheah I.K.-M., Yew T.S.K., Halliwell B. Distribution and accumulation of dietary ergothioneine and its metabolites in mouse tissues. Sci. Rep. 2018;8:1–15. doi: 10.1038/s41598-018-20021-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benoff S. Receptors and channels regulating acrosome reactions. Hum. Fertil. (Camb.) 1999;2:42–55. doi: 10.1080/1464727992000198311. [DOI] [PubMed] [Google Scholar]
- 64.Darszon A., Labarca P., Nishigaki T., Espinosa F. Ion channels in sperm physiology. Physiol. Rev. 1999;79:481–510. doi: 10.1152/physrev.1999.79.2.481. [DOI] [PubMed] [Google Scholar]
- 65.Benoff S., Hurley I., Cooper G.W., Mandel F.S., Rosenfeld D.L., Hershlag A. Head-specific mannose-ligand receptor expression in human spermatozoa is dependent on capacitation-associated membrane cholesterol loss. Hum. Reprod. 1993;8:2141–2154. doi: 10.1093/oxfordjournals.humrep.a137996. [DOI] [PubMed] [Google Scholar]
- 66.Benoff S., Cooper G.W., Centola G.M., Jacob A., Hershlag A., Hurley I.R. Metal ions and human sperm mannose receptors. Andrologia. 2000;32:317–329. doi: 10.1046/j.1439-0272.2000.00401.x. [DOI] [PubMed] [Google Scholar]
- 67.Wang R., Song B., Wu J., Zhang Y., Chen A., Shao L. Potential adverse effects of nanoparticles on the reproductive system. Int. J. Nanomed. 2018;13:8487–8506. doi: 10.2147/IJN.S170723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lansman J.B. Blockade of current through single calcium channels by trivalent lanthanide cations. Effect of ionic radius on the rates of ion entry and exit. J. Gen. Physiol. 1990;95:679–696. doi: 10.1085/jgp.95.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thill A., Zeyons O., Spalla O., Chauvat F., Rose J., Auffan M., Flank A.M. Cytotoxicity of CeO2 Nanoparticles for Escherichia coli. Physico-Chemical Insight of the Cytotoxicity Mechanism. Environ. Sci. Technol. 2006;40:6151–6156. doi: 10.1021/es060999b. [DOI] [PubMed] [Google Scholar]
- 70.Zeyons O., Thill A., Chauvat F., Menguy N., Cassier-Chauvat C., Oréar C., Daraspe J., Auffan M., Rose J., Spalla O. Direct and indirect CeO2 nanoparticles toxicity for Escherichia coli and Synechocystis. Nanotoxicology. 2009;3:284–295. doi: 10.3109/17435390903305260. [DOI] [Google Scholar]
- 71.Plakhova T.V., Romanchuk A.Y., Yakunin S.N., Dumas T., Demir S., Wang S., Minasian S.G., Shuh D.K., Tyliszczak T., Shiryaev A.A., et al. Solubility of Nanocrystalline Cerium Dioxide: Experimental Data and Thermodynamic Modeling. J. Phys. Chem. C. 2016;120:22615–22626. doi: 10.1021/acs.jpcc.6b05650. [DOI] [Google Scholar]
- 72.Lishko P.V., Botchkina I.L., Fedorenko A., Kirichok Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell. 2010;140:327–337. doi: 10.1016/j.cell.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 73.Mehrabi K., Nowack B., Arroyo Rojas Dasilva Y., Mitrano D.M. Improvements in Nanoparticle Tracking Analysis To Measure Particle Aggregation and Mass Distribution: A Case Study on Engineered Nanomaterial Stability in Incineration Landfill Leachates. Environ. Sci. Technol. 2017;51:5611–5621. doi: 10.1021/acs.est.7b00597. [DOI] [PubMed] [Google Scholar]
- 74.Xia T., Kovochich M., Brant J., Hotze M., Sempf J., Oberley T., Sioutas C., Yeh J.I., Wiesner M.R., Nel A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6:1794–1807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- 75.Singh N., Manshian B., Jenkins G.J.S., Griffiths S.M., Williams P.M., Maffeis T.G.G., Wright C.J., Doak S.H. NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials. 2009;30:3891–3914. doi: 10.1016/j.biomaterials.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 76.Ema M., Kobayashi N., Naya M., Hanai S., Nakanishi J. Reproductive and developmental toxicity studies of manufactured nanomaterials. Reprod. Toxicol. 2010;30:343–352. doi: 10.1016/j.reprotox.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 77.Blum J.L., Xiong J.Q., Hoffman C., Zelikoff J.T. Cadmium Associated With Inhaled Cadmium Oxide Nanoparticles Impacts Fetal and Neonatal Development and Growth. Toxicol. Sci. 2012;126:478–486. doi: 10.1093/toxsci/kfs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gao G., Ze Y., Li B., Zhao X., Zhang T., Sheng L., Hu R., Gui S., Sang X., Sun Q., et al. Ovarian dysfunction and gene-expressed characteristics of female mice caused by long-term exposure to titanium dioxide nanoparticles. J. Hazard. Mater. 2012;243:19–27. doi: 10.1016/j.jhazmat.2012.08.049. [DOI] [PubMed] [Google Scholar]
- 79.Tassinari R., Cubadda F., Moracci G., Aureli F., D’Amato M., Valeri M., De Berardis B., Raggi A., Mantovani A., Passeri D., et al. Oral, short-term exposure to titanium dioxide nanoparticles in Sprague-Dawley rat: Focus on reproductive and endocrine systems and spleen. Nanotoxicology. 2014;8:654–662. doi: 10.3109/17435390.2013.822114. [DOI] [PubMed] [Google Scholar]
- 80.Pietroiusti A., Massimiani M., Fenoglio I., Colonna M., Valentini F., Palleschi G., Camaioni A., Magrini A., Siracusa G., Bergamaschi A., et al. Low doses of pristine and oxidized single-wall carbon nanotubes affect mammalian embryonic development. ACS Nano. 2011;5:4624–4633. doi: 10.1021/nn200372g. [DOI] [PubMed] [Google Scholar]
- 81.Williams R., Sparacino C., Petersen B., Bumgarner J., Jungers R.H., Lewtas J. Comparative characterization of organic emissions from diesel particles, coke oven mains, roofing tar vapors and cigarette smoke condensate. Int. J. Environ. Anal. Chem. 1986;26:27–49. doi: 10.1080/03067318608077102. [DOI] [PubMed] [Google Scholar]
- 82.Scheepers P.T.J., Bos R.P. Combustion of diesel fuel from a toxicological perspective. Int. Arch. Occup. Environ. Heath. 1992;64:149–161. doi: 10.1007/BF00380904. [DOI] [PubMed] [Google Scholar]
- 83.Brevik A., Lindeman B., Rusnakova V., Olsen A.-K., Brunborg G., Duale N. Paternal Benzo[a]pyrene Exposure Affects Gene Expression in the Early Developing Mouse Embryo. Toxicol. Sci. 2012;129:157–165. doi: 10.1093/toxsci/kfs187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.