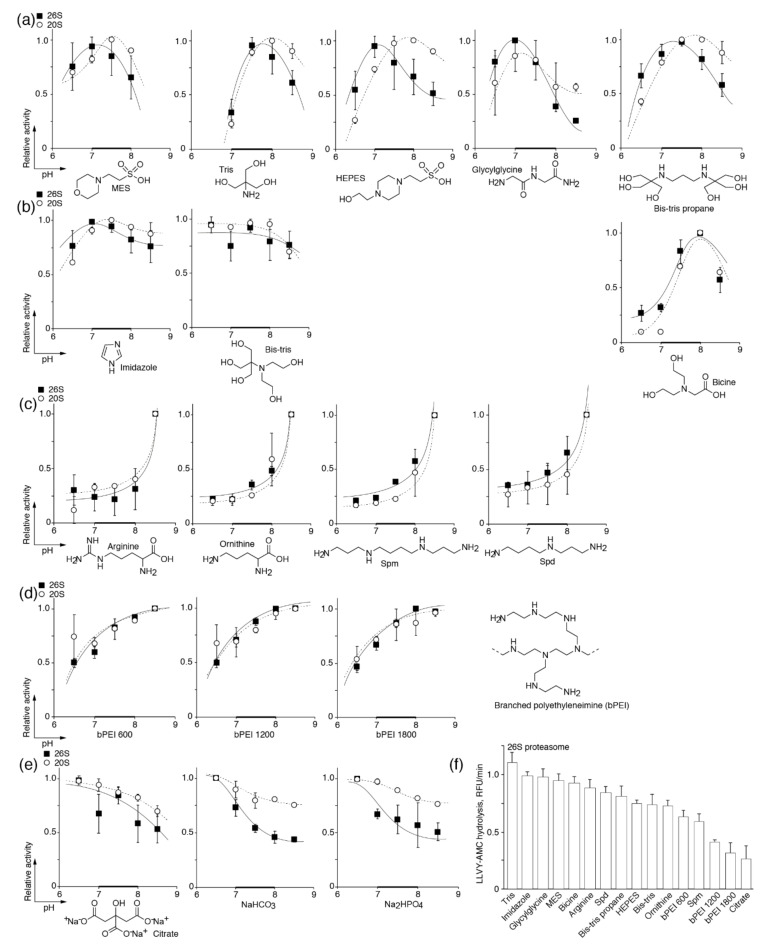
Figure 2.
The pH dependence of the proteasomal chymotrypsin-like peptidase activity in different buffers containing (poly)amines (a–d) and anionic buffers (e). The skewed distribution (a), pH-independent (b), bursting (c) and progressive (d) types of activation are shown. Activity levels of the 26S (■) and 20S (○) proteasomes were measured using succinyl-Leu-Leu-Val-Tyr-7-amido-4-methylcoumarin (Suc-LLVY-AMC) at 25 mM of each buffer system. Relative activity was calculated as the ratio of activity at a distinct pH to the maximal activity within the tested pH range. (f) Absolute activity of the 26S proteasome in different buffers at the optimal pH value for each buffer system. The data represent the average and standard deviation (error bars) from four independent measurements. Natural polyamines (Spm, Spd) and branched polyamines (bPEI) were used at a concentration of 5 mM; all other buffers were at 25 mM. The data were fitted to a polynomial square of an exponential or Gauss function. The physiologically relevant pH range is shown by a bold line.