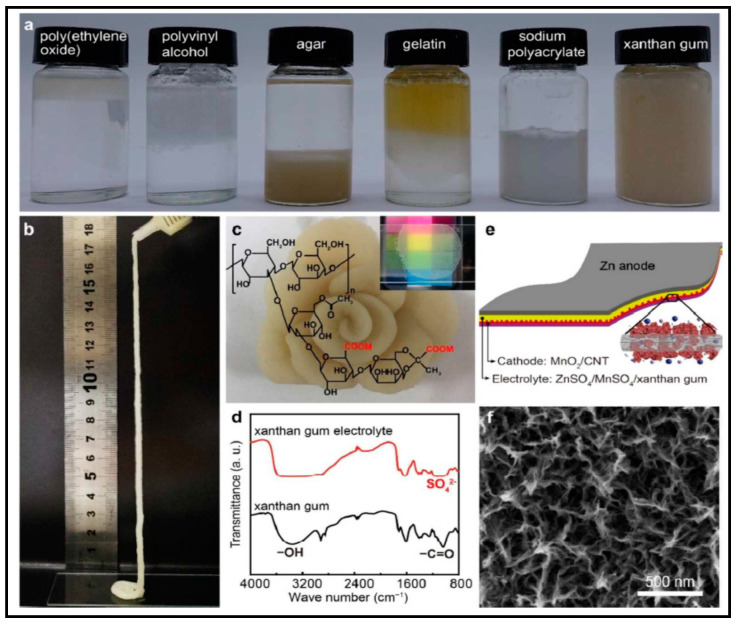
Figure 21.
(a) Photograph of aqueous solutions of 2 M ZnSO4 and 0.1 MnSO4 after adding 10 wt.% polymers of poly(ethylene oxide) (PEO), polyvinyl alcohol (PVA), agar, gelatine, sodium polyacrylate and xanthan gum showing that PEO, PVA, agar, gelatine and sodium polyacrylate were aggregated while xanthan gum was uniformly dissolved in the solution. (b) Photograph showing that the sticky xanthan gum electrolyte could extruded from a syringe and self-support at a long length. (c) A hand-made flower using the xanthan gum electrolyte, the molecular formula of xanthan gum floating on the flower, and the top-right photo showing that the xanthan gum electrolyte can be readily shaped into a 10-μm-thick film. (d) IR spectra of xanthan gum and the xanthan gum electrolyte. (e) Schematic showing the structure of our gum Zn–MnO2 battery, which consists of a zinc foil as the anode, a ZnSO4/MnSO4/xanthan gum film as the separator and the electrolyte, and a MnO2/CNT film as the cathode, respectively. The pop-up shows the structure of the MnO2/CNT electrode, where a porous MnO2 layer is coated on a CNT thin-film. (f) SEM image of the MnO2/CNT hybrid film. Reproduced with permission from [64]. Copyright 2018 Royal Society of Chemistry.