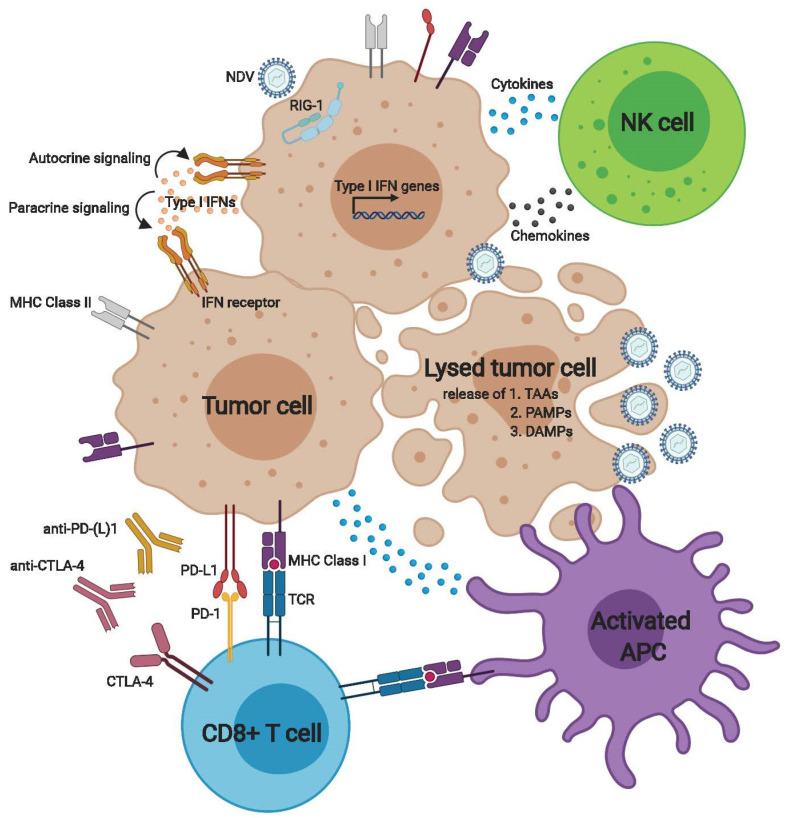
Figure 1.
Newcastle disease virus (NDV) activates innate and adaptive anti-tumor immune responses. NDV selectively infects tumor cells that have defective anti-viral defenses. Extracellular and intracellular signaling mediated by sensors such as the RNA helicase RIG-1 leads to expression of type I IFN and related genes. Autocrine and paracrine IFN signaling upregulates MHC class I and II presentation, co-stimulatory molecules, and immune checkpoints on the cell surface. The release of cytokines and chemokines in addition results in the recruitment of innate effector cells such as NK cells and macrophages and antigen-presenting cells (APCs). Virus-mediated direct oncolysis leads to release of tumor antigens, PAMPs, and DAMPs that activate APCs including dendritic cells capable of antigen cross-presentation. Activated APCs prime T cells, resulting in generation of cytolytic T cells directed toward tumor and viral antigens; however, effector function of the activated T cells can be inhibited by upregulation of PD-L1 on tumor cells and APCs, and PD-1 and CTLA-4 on T cells. Upregulation of these negative feedback mechanisms provide the rationale for combining NDV with immune checkpoint inhibitors.