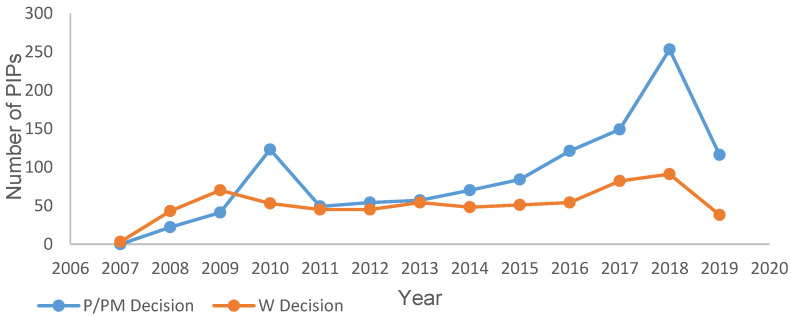
Figure 3.
Number of positive (P/PM) PIP decisions and waivers (W) granted per year by the PDCO, since the EUPR was established in 2007. Data from 2007 and 2019 correspond to incomplete years (February to December 2007 and January to October 2019). Data taken from EMA repository [20]. See methods for details on data mining. P: decision agreeing on an investigation plan with or without partial waiver(s) and or deferral(s); PM: decision on the application for modification of an agreed PIP; W: decision granting a waiver in all age groups for all conditions/indications.