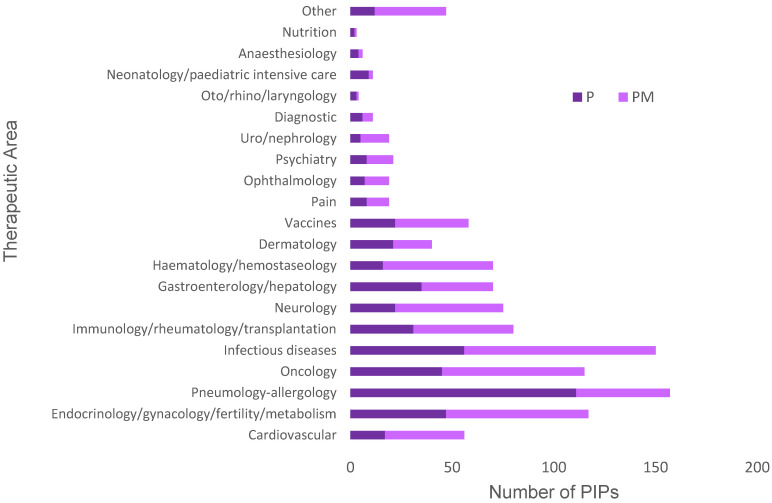
Figure 5.
Number of positive decisions (P or PM) granted by the PDCO since the establishment of the EUPR in 2007 for the EMA therapeutic areas. Data taken from EMA repository [20]. See methods for details on data mining. PIP: paediatric investigation plan; PDCO: Paediatric Committee; EUPR: European Union Paediatric Regulation; P: decision agreeing on an investigation plan with or without partial waiver(s) and or deferral(s); PM: decision on the application for modification of an agreed PIP.