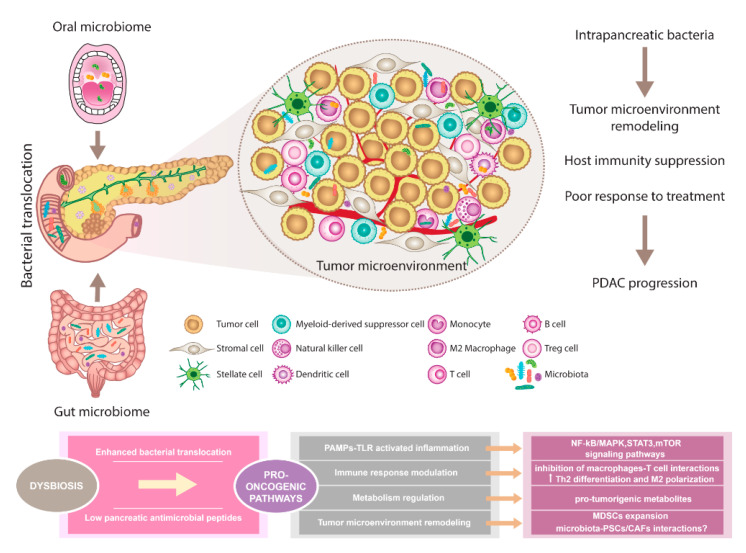
Figure 1.
The pancreatic microbiota as a component of the tumor microenvironment. Comprehensive microbial studies support the existence of constant interactions between oral, gut, and pancreatic microbiome. Dysbiosis contributes to multiple changes associated with PDAC. Disruption of gut microbiota leads to enhanced bacterial translocation and subsequent activation of TLRs signaling pathway in the pancreatic environment. Besides, decreased SCFA metabolites negatively influence the production of pancreatic antimicrobial peptides. Microbiota-derived signals might affect pancreatic oncogenesis via NF-kB/MAPK, STAT3, or mTOR tumor-related inflammatory pathways. Moreover, immune response modulation leading to reduced Th1 CD4+ and CD8+ T cell differentiation and increased Th2 levels as well as the production of pro-tumorigenic metabolites might represent the pro-oncogenic mechanisms. Since microbiota has been recognized as an important part of the PDAC microenvironment, possible interactions with PSCs/CAFs need to be considered and further evaluated. Importantly, microbiota-dependent remodeling of tumor microenvironment towards PDAC immunosuppression has been reported, suggesting the complex interplay between all components within the tumor might affect the sensitivity to PDAC treatment. Abbreviations: CAFs, cancer-associated fibroblasts; MAPK, a mitogen-activated protein kinase; MDSCs, myeloid-derived suppressor cells; mTOR, the mammalian target of rapamycin; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; PAMPs, pathogen-associated molecular patterns; PDAC, pancreatic ductal adenocarcinoma; PSCs, pancreatic stellate cells; SCFA, short-chain fatty acid; STAT3, signal transducer and activator of transcription 3; Th2 cells, T helper 2 cells; T reg cells, regulatory T cells; TLRs, Toll-like receptors.