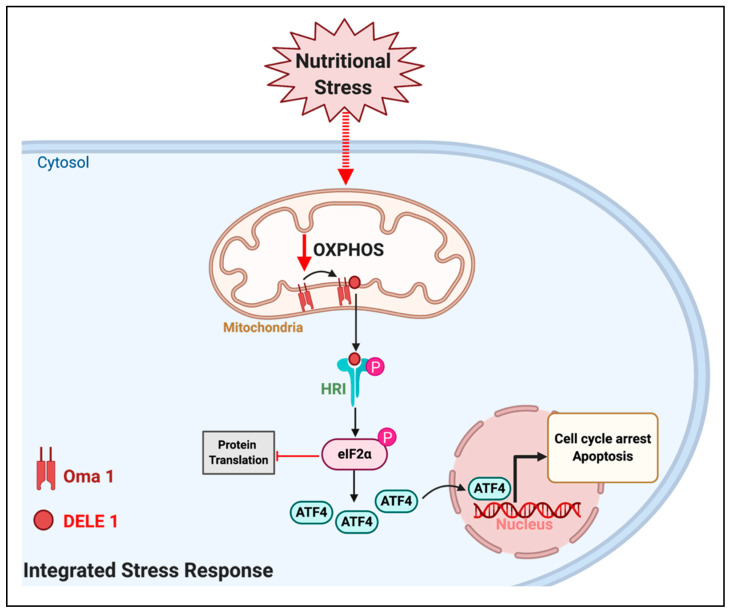
Figure 3.
The effects of extracellular nutrients on mitochondria could activate the integrated stress response (ISR) pathway in cancer. Changes in extracellular nutrient availability can induce changes in mitochondrial function leading to decreased oxidative phosphorylation (OXPHOS), namely mitochondrial ATP synthesis fueled by respiration. A decrease in OXPHOS can activate the mitochondrial protease OMA1, which cleaves the mitochondrial protein DELE1. The short form of DELE 1 can be released to the cytosol, bind to the Heme-regulated inhibitor kinase (HRI) and activate its function. HRI phosphorylates the eukaryotic translation initiation factor 2a (eIF2 a), which inhibits protein translation, with the exception of the transcription factor ATF4. The actions of ATF4 in the nucleus execute the ISR to induce cell cycle arrest and even apoptosis.