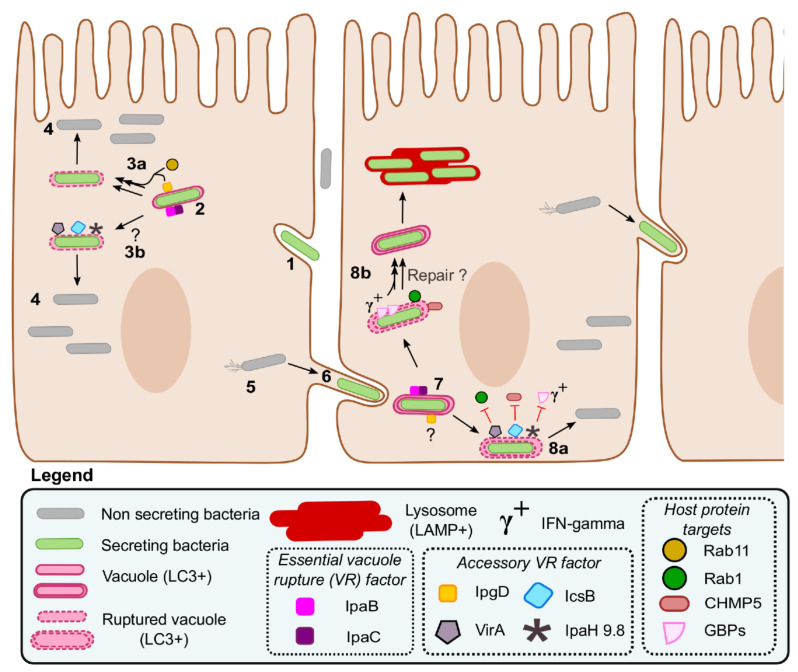
Figure 5.
Shigella intracellular lifestyle and role of its virulence factors during vacuole escape. The invasion of a nonphagocytic host cell starts by the activation of T3SAs, which induce the remodeling of the plasma membrane through the action of their translocators and effectors (1). The intracellular bacterium is captured in a single membrane entry vacuole; several T3SA substrates, including the translocators IpaB, IpaC, and IpgD, accumulate in the vicinity of the vacuole (2). IpaBC form pores that are essential to vacuole rupture; the IpgD-dependent recruitment of Rab11 might accelerate this process (3a); although the other accessory vacuole rupture factors IcsB, VirA, and IpaH9.8 are dispensable during entry in epithelial cells, they might be important in yet undiscovered conditions such as in immune cells or in specific cytokinic contexts (3b). Cytosolic bacteria proliferate (4) and move using actin comets (5). Upon contact with the plasma membrane, the T3SA is reactivated, allowing the formation of a protrusion (6) and a double membrane dissemination vacuole (7). IpaB and IpaC initiate the rupture of the double membrane dissemination vacuole; the role of IpgD at this stage is unknown. The accessory VR factors IcsB and VirA, and probably IpaH9.8 in the presence of interferon-gamma (IFNγ), facilitate the escape of Shigella from the dissemination vacuole already partly ruptured by IpaBC (8a); this is probably realized through the inhibition of their respective targets CHMP5, Rab1, and GBPs. These WT bacteria can then resume cell-to-cell spread. By contrast, in the absence of these accessory VR factors, the repair of the partially ruptured vacuole is induced by Rab1 and CHMP5 (8b), a process that might also be facilitated by the action of the GBPs when IFNγ is present. These mutants (8b) are more often captured in lysosomes and partly deficient in cell-to-cell spread.